
Confidential Draft Submission No. 2 submitted to the Securities and Exchange Commission on March 12, 2021. This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Confidential Draft Submission No. 2
FORM S-1
REGISTRATION STATEMENT
under the Securities Act of 1933
Anebulo Pharmaceuticals, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware (State or Other Jurisdiction of Incorporation or Organization) |
2834 (Primary Standard Industrial Classification Number) |
85-1170950 (I.R.S. Employer Identification No.) |
Anebulo Pharmaceuticals, Inc.
1415 Ranch Road 620 South, Suite 201
Lakeway, Texas 78734
(512) 598-0931
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Daniel Schneeberger, M.D.
Chief Executive Officer
Anebulo Pharmaceuticals, Inc.
1415 Ranch Road 620 South, Suite 201
Lakeway, Texas 78734
(512) 598-0931
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Spencer
G. Feldman, Esq. Olshan Frome Wolosky LLP 1325 Avenue of the Americas, 15th Floor New York, New York 10019 (212) 451-2300 |
Ben A. Stacke, Esq. Jonathan R. Zimmerman, Esq. Faegre Drinker Biddle & Reath LLP 2200 Wells Fargo Center 90 S. Seventh Street Minneapolis, Minnesota 55402 (612) 766-7000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. [ ]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer [ ] | Accelerated Filer [ ] | Non-Accelerated Filer [X] | Smaller Reporting Company [X] Emerging Growth Company [X] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. [ ]
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee(3) | ||||||
| Common Stock, par value $0.001 per share | $ | $ | ||||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Includes the aggregate offering price of additional shares that the underwriters have the option to purchase to cover over-allotments, if any. |
| (3) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state or jurisdiction where the offer or sale of these securities is not permitted.
SUBJECT TO COMPLETION, DATED , 2021
PRELIMINARY PROSPECTUS
Shares

Anebulo Pharmaceuticals, Inc.
Common Stock
This is the initial public offering of common stock of Anebulo Pharmaceuticals, Inc. We are offering shares of our common stock. Prior to this offering, no public market has existed for our common stock. We anticipate that the initial public offering price will be between $ and $ per share of common stock. We intend to apply to list our common stock for trading on The Nasdaq Capital Market under the symbol “ANEB.”
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 14 of this prospectus to read about factors you should consider before buying shares of our common stock.
| Per Share | Total | |||||||
| Initial public offering price | $ | $ | ||||||
| Underwriting discounts and commissions(1) | $ | $ | ||||||
| Proceeds, before expenses, to us | $ | $ | ||||||
| (1) | In addition, we have agreed to reimburse the underwriters for certain expenses. Please see the section of this prospectus entitled “Underwriting” for additional information regarding underwriters’ compensation. |
We have granted the underwriters an option to purchase up to additional shares of common stock from us at the initial public offering price less underwriting discounts and commissions to cover over-allotments, if any. The underwriters can exercise this option within 30 days after the date of this prospectus.
We are an “emerging growth company” as defined under U.S. federal securities laws and, as such, may elect to comply with certain reduced public company reporting requirements after this offering.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of our common stock to purchasers on or about , 2021, subject to customary closing conditions.
The Benchmark Company
The date of this prospectus is , 2021.
TABLE OF CONTENTS
About this Prospectus
Neither we nor the underwriters have authorized anyone to provide you with information that is different from that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are offering to sell shares of common stock and seeking offers to buy shares of common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date on the front of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: Neither we nor the underwriters have done anything that would permit this offering, or possession or distribution of this prospectus, in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus outside of the United States. See “Underwriting.”
Unless otherwise indicated, information in this prospectus concerning economic conditions, our industry, our markets and our competitive position is based on a variety of sources, including information from third-party industry analysts and publications and our own estimates and research. Some of the industry and market data contained in this prospectus are based on third-party industry publications. This information involves a number of assumptions, estimates and limitations. The sources of the third-party industry publications referred to in this prospectus are:
| ● | The United States Census Bureau; and | |
| ● | The Nationwide Emergency Department Sample (“NEDS”). |
The industry publications, surveys and forecasts and other public information generally indicate or suggest that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. None of the third-party industry publications used in this prospectus were prepared on our behalf. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause results to differ materially from those expressed in these publications.
“Anebulo” and other registered or common law trade names, trademarks, or service marks of Anebulo Pharmaceuticals, Inc. appearing in this prospectus are the property of Anebulo Pharmaceuticals, Inc. This prospectus contains additional trade names, trademarks, and service marks of other companies that are the property of their respective owners. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, these other companies. Solely for convenience, our trademarks and trade names referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extend under applicable law, our rights, or the right of the applicable licensor, to these trademarks and trade names.
| i |
This summary highlights selected information that is presented in greater detail elsewhere in this prospectus and does not contain all of the information that you should consider before investing in our common stock. Before investing in our common stock, you should read this entire prospectus carefully, including the information set forth under the sections “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes thereto, in each case included in this prospectus. Some of the statements in this prospectus constitute forward-looking statements. See “Cautionary Note Regarding Forward-Looking Statements.”
Unless the context requires otherwise, the words “we,” “us,” “our,” “our company” and “our business” refer to Anebulo Pharmaceuticals, Inc., a Delaware corporation.
Our Company
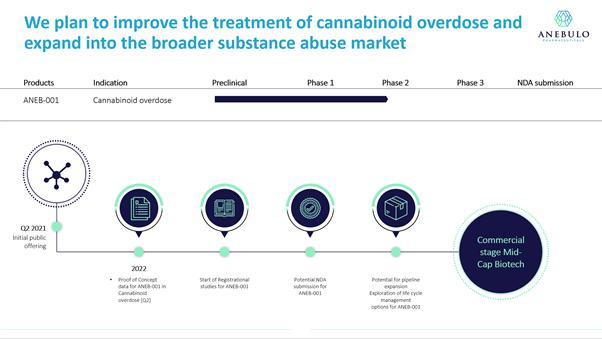
We are a clinical-stage biotechnology company developing novel solutions for people suffering from cannabinoid overdose and substance addiction. Our lead product candidate, ANEB-001, is intended to reverse the negative effects of cannabinoid overdose within 1 hour of administration. The signs and symptoms of cannabinoid overdose range from profound sedation to anxiety and panic to psychosis with hallucinations. There is no approved medical treatment currently available to specifically alleviate the symptoms of cannabinoid overdose. If approved by the U.S. Food and Drug Administration (the “FDA”), we believe ANEB-001 has the potential to be the first FDA approved treatment of its kind on the market for reversing the effects of tetrahydrocannabinol (“THC”), the principal psychoactive constituent of cannabis. Clinical trials completed to date have shown that ANEB-001 is rapidly absorbed, well tolerated and leads to weight loss, an effect that is consistent with central cannabinoid receptor type 1 (“CB1”) antagonism. We intend to launch a Phase 2 proof - of - concept trial for cannabinoid overdose in the fourth quarter of 2021.
Cannabinoid overdoses have become a widespread health issue in the United States, particularly in the increasing number of states that have legalized cannabis for personal and recreational use. In recent years, hospital emergency rooms across the United States have seen a dramatic increase in patient visits with cannabis-related conditions. Before the legalization of cannabis, an estimated 450,000 patients visited hospital emergency rooms for cannabis-related conditions. In 2014, this number more than doubled to an estimated 1.1 million patients, according to data published in “Trends and Related Factors of Cannabis-Associated Emergency Department Visits in the United States: 2006-2014,” Journal of Addiction Medicine (May/June 2019), which provided a national estimate analyzing data from The Nationwide Emergency Department Sample (“NEDS”), the largest database of U.S. hospital-owned emergency department visits. Based on our own analysis of the most recent NEDS data, we believe that the number of hospitalizations grew to 1.74 million patients in 2018 and was growing at an approximately 15% compounded annual growth rate between 2012 and 2018. We believe the number of cannabis-related hospitalizations and other health problems associated with cannabinoid overdoses such as depression, anxiety and mental disorders will continue to increase substantially as more states pass laws legalizing cannabis for medical and recreational use. Given the consequences, there is an urgent need for a treatment to rapidly reverse the symptoms of cannabinoid overdose.
The ingestion of large quantities of tetrahydrocannabinol is a major cause of cannabinoid overdose. Excessive ingestion of THC via edible products such as candies and brownies, and overdoses of synthetic cannabinoids (also known as “synthetics,” “K2” or “spice”), are two leading causes of THC-related emergency room visits. Synthetic cannabinoids are analogous to fentanyl for opioids insofar as they are more potent at the cannabinoid receptor than their natural product congener THC. Individuals can use or consume cannabinoids in natural or unnatural formulations, orally or by inhalation, and intentionally and unintentionally, all of which can result in an overdose. Natural formulations include edibles and marijuana cigarettes and unnatural formulations include synthetics. Individuals consume cannabinoids orally by ingesting edibles or synthetics and by inhalation through smoking marijuana cigarettes or synthetics. Cannabinoids can also be ingested unintentionally through these same methods where, for example, children consume edibles by mistaking them for common consumer items like candy that would not otherwise contain THC. Symptoms of cannabinoid overdoses produced by edibles and synthetics can include psychosis, panic and anxiety, feelings of paranoia, agitation, hallucinations, nausea, vomiting, cardiac arrhythmias, seizures and death. Many of these symptoms can require emergency medical attention and can take hours to days to resolve depending on the particular product and amount ingested. Currently, there is no specific treatment to reverse cannabis overdose and physicians have to rely on supportive care, including benzodiazepines, and wait for the body to metabolize the THC or synthetic cannabinoid.
We were founded in April 2020, and in May 2020 we entered into an exclusive worldwide license agreement with Vernalis (R&D) Limited (“Vernalis”), a drug discovery subsidiary of Ligand Pharmaceuticals Incorporated, to develop and commercialize ANEB-001. Vernalis has been the sponsor of all prior preclinical and clinical studies. Since the in-licensing with Vernalis, we have assembled an executive team and started preparations for a Phase 2 proof-of-concept trial, including the synthesis of a new active pharmaceutical ingredient (“API”), and the development and filing of a clinical trial protocol with regulatory agencies in Europe. We are in the process of obtaining patents intended to cover our product, composition and methods of use that are important to the development of our business. We have filed two patent applications for various methods of use of the ANEB-001 compound and delivery systems, which applications are currently pending before the U.S. Patent and Trademark Office.
| 1 |
Our Lead Product Candidate
Our objective is to develop and commercialize new treatment options for patients suffering from cannabinoid overdose and addiction. Our lead product candidate is ANEB-001, a potent, small molecule cannabinoid receptor antagonist, designed to address the unmet medical need for a specific antidote for cannabinoid overdose. CB1 antagonists bind to the CB-1 receptor and thereby reverse the action of cannabinoids such as THC. ANEB-001 is an orally bioavailable, rapidly absorbed treatment that we anticipate will reverse the symptoms of cannabinoid overdoses, in most cases within 1 hour of administration. Phase 1 trials showed that ANEB-001 is rapidly absorbed, well tolerated and leads to weight loss, an effect that is consistent with central CB1 antagonism. We intend to commence starting a Phase 2 proof - of - concept trial for ANEB-001 in the fourth quarter of 2021. Our proprietary position in the treatment of cannabinoid overdose is protected by rights to two patent applications covering various methods of use of the compound and delivery systems.
Our Market Opportunity
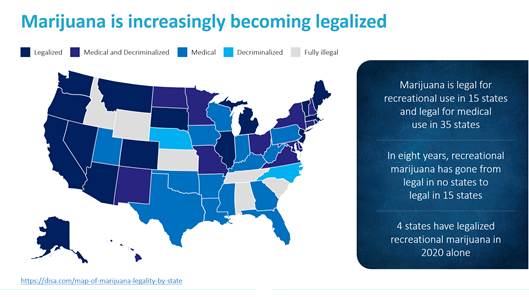
Cannabinoid overdoses have become a widespread health issue in the United States as an increasing number of states have legalized cannabis for personal and recreational use. As of December 31, 2020, cannabis was legal for recreational use in 15 states and legal for medical use in 35 states. Additionally, the Centers for Disease Control and Prevention and recent news reports have described how the stress, anxiety and depression from the prolonged stay-at-home conditions surrounding the Covid-19 pandemic appears to be resulting in excessive drug and cannabis use by individuals, whether in jurisdictions where such use is legal or not.
| 2 |
Cannabinoid overdoses frequently occur due to the ingestion of edibles, which can contain relatively large amounts of THC, and consumption of synthetics. Symptoms of cannabinoid overdoses produced by edibles and synthetics can include psychosis, panic and anxiety, feelings of paranoia, agitation, hallucinations, nausea, vomiting, cardiac arrhythmias, seizures and death. These symptoms can require emergency medical attention and can take hours to days to resolve. According to an article published in the Journal of Addiction Medicine that analyzed data from NEDS, an estimated 1.1 million emergency department visits were associated with cannabis in 2014. We have performed our own independent analysis of all currently available NEDS datasets and estimated that the number of cannabis-associated emergency department visits increased to 1.74 million patients in 2018. The number of cannabis-associated emergency department visits has grown at a 15% compounded annual growth rate from 2012 to 2018, which is when states first began legalizing recreational cannabis use.
| 3 |
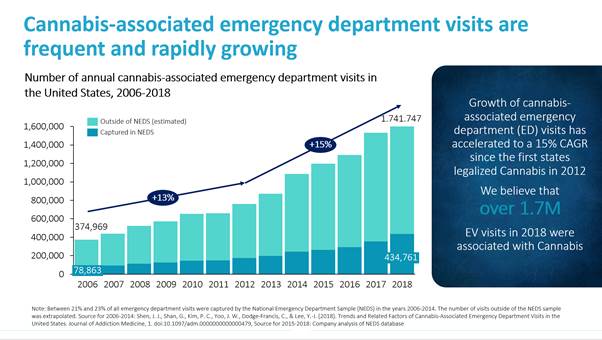
Source for 2006-2014: Shen, J. J., Shan, G., Kim, P. C., Yoo, J. W., Dodge-Francis, C., & Lee, Y.-J. (2018). Trends and Related Factors of Cannabis-Associated Emergency Department Visits in the United States. Journal of Addiction Medicine, 1. doi:10.1097/adm.0000000000000479, Source for 2015-2018: Company analysis of NEDS database.
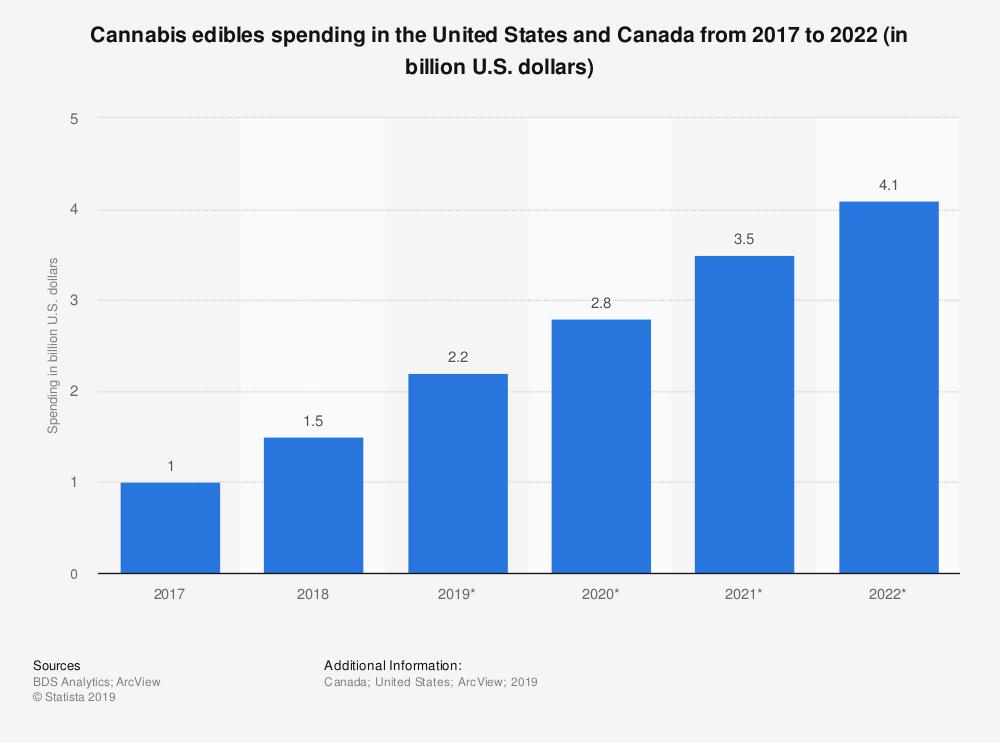
We believe that both the number of cannabis-associated emergency department visits and the unmet medical need will continue to grow due to the increasing popularity of edibles. In THC-containing edibles, the median dose of THC can be many times more potent than the recommended safe dosage and as much as 8 times more potent than a rolled marijuana cigarette. Edibles are frequently manufactured as common consumer products, such as brownies, cookies, candies and gummy snacks with brightly-colored packaging. THC concentrations in edibles peak after a delay of about 2 to 4 hours from ingestion. This contrasts with smoking cannabis, which causes THC concentrations to peak in about 3 to 10 minutes from inhalation. Consumers are likely to approach edibles with the same serving size expectations as consumer products without THC. Moreover, children are particularly at risk for accidentally consuming edibles due to their brightly-colored packaging and formulation into candies and sweets. The confluence of these factors can be dangerous and increases the risk of cannabinoid overdose. Emergency department visits were 33 times more likely for edibles as compared with other routes of cannabis consumption, according to the recent article “Mental Health-related Emergency Department Visits Associated with Cannabis in Colorado,” published in Academic Emergency Medicine (May 2018). Sales of edibles are rapidly growing, according to data collected by Statista, and are expected to continue growing for the foreseeable future.
In November 2020, we engaged a consultation and data services provider to conduct a survey of U.S. physicians concerning patient emergency room visits for cannabinoid overdoses within the past 12 months. Based on its survey of 27 emergency room physicians throughout the United States, the survey provider reported that the surveyed physicians saw on average 10.5 patients (a range of 2 to 45 patients) with cannabis intoxication per month. The survey asked these physicians to rank on a scale of 1 to 10 (i) the need for a cannabinoid antagonist to treat cannabis intoxication; (ii) the likelihood of their prescribing a cannabinoid antagonist that reverses cannabis intoxication within 30 minutes of administration; and (iii) the likelihood of such cannabinoid antagonist reducing the need for supportive medication to manage certain cannabis intoxication symptoms, such as agitation and acute psychosis. In response to these questions, the surveyed physicians ranked the need for a cannabinoid antagonist at an average of 7.52 out of 10, the likelihood of prescribing a cannabinoid antagonist that reverses cannabis intoxication within 30 minutes of administration at an average of 7.44 out of 10, and the likelihood of a specific cannabinoid antagonist reducing the need for supportive medication to manage certain cannabinoid overdose symptoms at an average of 7.48 out of 10.
| 4 |
We believe that the market opportunity for our lead product candidate, ANEB-001, will continue to expand and accelerate if additional states pass laws to legalize recreational cannabis use. On December 4, 2020, the U.S. House of Representatives voted in favor of a bill to decriminalize marijuana at the federal level by removing cannabis from the list of controlled substances under the Controlled Substances Act. Although it is currently uncertain whether this bill will be subsequently approved by the U.S. Senate and signed into law by the President, in the event the use of cannabis is legalized in the United States at the federal level, we believe that the greater anticipated number of users will significantly increase the potential need for our lead candidate.
We believe that overdose due to synthetic cannabinoids is an area with particularly high unmet medical need. Synthetics are among the fastest growing class of psychoactive drugs worldwide and can be as much as 85 times as potent as THC. Unlike edibles and other cannabis products, synthetics have low shipping weights and can more readily evade traditional drug screening methods. This likely reflects the structural promiscuity of the CB1 receptor. In addition, the negative effects of an overdose from synthetics can be longer lasting and more severe when compared with THC. These negative effects could include seizures, and even death.
Our Growth Strategy
Our goal is to create a therapeutic to treat the symptoms of cannabinoid overdose and substance addiction. As noted above, there are currently no FDA approved medical treatments on the market to specifically alleviate the negative psychological effects of cannabinoid overdose. The absence and growing unmet need for such a treatment gives us the unique opportunity to create a novel solution and become a leader in the cannabinoid treatment space. To achieve our goal, our strategy will be guided by the following principles:
| ● | Develop and commercialize our ANEB-001 antagonist in the United States. We anticipate commencing our Phase 2 proof-of-concept study in the fourth quarter of 2021. We believe the data from this study may facilitate discussions of a regulatory path for ANEB-001 in the United States. | |
| ● | Explore strategic collaborations to commercialize ANEB-001. Our plan is to widely commercialize ANEB-001. To accomplish this objective, we may partner with companies that possess a direct sales force and sales representatives. | |
| ● | Strive for capital efficiency in developing ANEB-001. We aim to be capital efficient in our development of ANEB-001 by outsourcing our clinical research and data management. We anticipate this will lower our clinical development costs and improve our ability to efficiently commercialize ANEB-001 once approved by the FDA. | |
| ● | Introduce promising product candidate extensions. We are in the initial stages of introducing a non-oral formulation of ANEB-001 that we intend to develop for the use in cannabinoid hyperemesis syndrome (CHS), which is a condition that can develop following long-term use of marijuana and is characterized by cyclical episodes of nausea and vomiting that are not usually responsive to standard care. We believe that antagonizing the paradox emetogenic action of THC at the receptor and helping patients abstain from THC represent the most promising and causal treatment for CHS. | |
| ● | Develop future product candidates to treat cannabinoid and substance-related addiction. We intend to leverage our expertise in the endocannabinoid system to develop additional product candidates for the treatment of substance addiction. CB1 antagonists have been shown to be promising in treating substance-related addiction. We believe that there is a large and growing unmet medical need for new treatment options because of the opioid and methamphetamine epidemic. |
Our Clinical Trials and Development Plan
We are developing ANEB-001 to quickly and effectively combat the symptoms of cannabinoid overdose. ANEB-001 is a competitive CB1 antagonist with a high affinity for the human CB1 receptor (0.6 nM). In vitro testing showed ANEB-001 had >1000x selectivity with the human CB1 receptor over all other tested receptors. As part of the preclinical characterization of ANEB-001, Vernalis demonstrated that oral administration of ANEB-001 reduced hypolocomotion in mice after 30 minutes, effectively reversing the actions of THC. In 2006 and 2007, two Phase 1 studies for the treatment of obesity were conducted by Vernalis for ANEB-001.
Phase 1a
The Phase 1a study (V24343-1Ob-01) administered single (Part A) and multiple (Part B) ascending doses of ANEB-001 for up to 14 days in otherwise healthy overweight and mildly obese subjects.
| ● | Part A randomized 18 healthy volunteers to receive either a placebo (n=18) or two single oral doses of ANEB-001, with doses ranging from 1 mg to 200 mg. No severe adverse events were observed in either group in Part A. There was no difference between treatment groups in Part A in overall incidence, number of or severity of adverse events. Probable drug-related events in the treatment arm were nausea (22%), dizziness (11%), hiccups (8%), and decreased appetite (8%). |
| 5 |
| ● | Part B randomized 32 obese volunteers to receive either a placebo (8 obese volunteers) or 4 different doses of ANEB-001 for 14 days (24 obese volunteers). No severe adverse events were observed in either group in Part B, but an increased number of mild and moderate adverse events was observed in the obese volunteers who received the 2 higher dose arms (200/50 mg and 100 mg). The observed adverse events included nausea, vomiting, diarrhea, dizziness, hiccups, decreased appetite, hyperhidrosis and feeling hot. We believe these adverse events are “on-target,” meaning they reflect CB1 antagonism, because these adverse events have also been observed with other CB1 antagonists. |
Pharmacokinetic measurements in Part A of the Phase 1a study demonstrated that ANEB-001 was rapidly absorbed by the body following oral administration and achieved blood concentrations anticipated to exceed those necessary to block the cannabinoid receptor (as indicated by the red line in the diagram below).
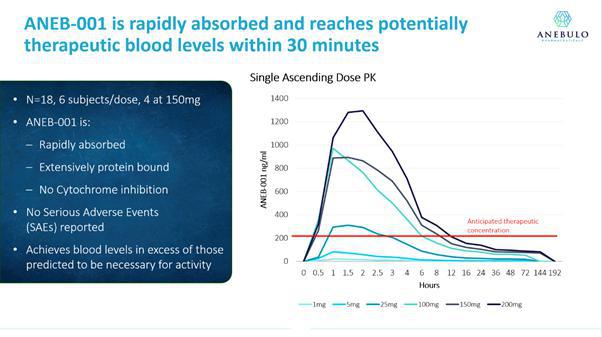
Vernalis also measured the impact of ANEB-001 on anxiety and depression in Part B of the Phase 1a study. Vernalis measured anxiety by using the Spielberger state score, a commonly used measure of trait and state anxiety. Vernalis found no significant impact on anxiety, except for the 200/50 mg arm, which showed increased anxiety at all assessment times. The change was driven by a single subject and may be explained by somatic adverse events, which contributed to the Spielberger score. For depression, HAMD21 was used and small increases were noted in the 75/15 mg and 200/50 mg dose, which we believe were likely driven by somatic symptoms.
Summarizing the results from the Phase 1a study, ANEB-001 doses between 1 mg and 150 mg were found to be very well tolerated in both single and multiple doses with an adverse events profile similar to the placebo. There was no observed effect on the cardiovascular system, ECGs, labs or physical exams and no significant effects on anxiety or depression scores.
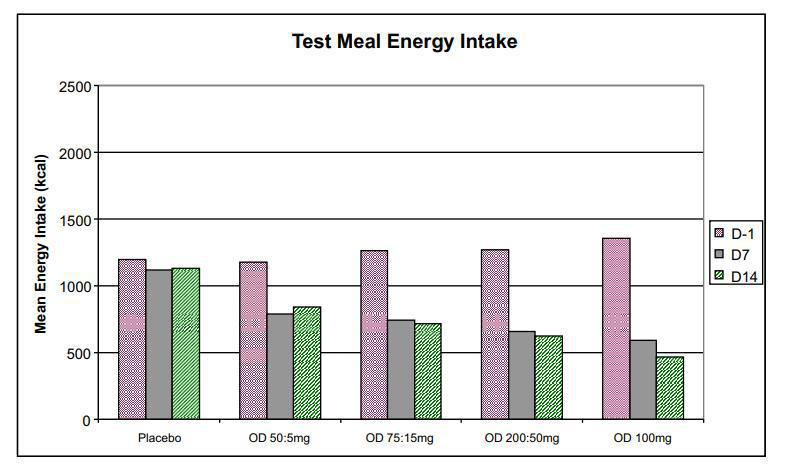
With regard to pharmacodynamics, a marked reduction in test meal energy intake was seen even at the lowest dose level in Phase 1a Part B. Further, Vernalis observed statistically significant decreases in body weight indicating that ANEB-001 was able to cross the blood-brain barrier and antagonize central cannabinoid receptors.
| 6 |
Phase 1b
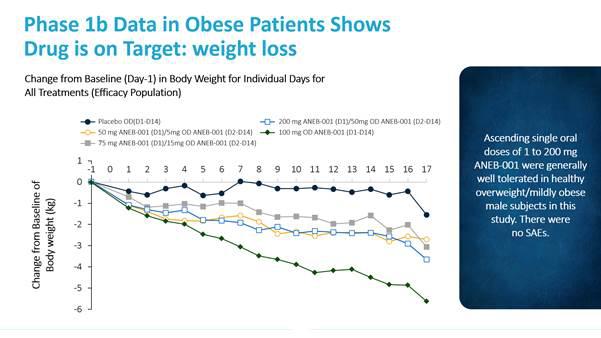
The Phase 1b study (V24343-1Ob-02) compared the pharmacokinetics of a single oral dose (1 to 200 mg) of ANEB-001 to subjects in fed and fasted states, and to subjects that were lean and overweight. There were no apparent differences in the tolerability of ANEB-001 between the subjects that were in fed and fasted states or subjects that were lean and overweight. Total AUC (or area under the curve) was approximately 30% higher in subjects in the fed state compared to the subjects in the fasted state, with similar systemic exposure for the lean and overweight subjects.
The results of the Phase 1 studies demonstrate that ANEB-001 was well-tolerated among healthy and obese subjects. There were no serious adverse events. The most commonly reported adverse event was gastrointestinal discomfort, which also occurred in subjects that were administered placebos. Based on the promising results of the Phase 1 studies, we believe ANEB-001 may offer the following clinical and product benefits:
| ● | Oral bioavailability. ANEB-001 will be available as an oral treatment in the form of a pill, capsule or tablet. |
| 7 |
| ● | Rapid absorption. We believe ANEB-001 can rapidly reverse the signs and symptoms of cannabinoid overdose in as little as 1 hour. | |
| ● | Low likelihood of drug-to-drug interactions. Preclinical testing demonstrated that ANEB-001 did not inhibit the metabolic enzymes cytochromes 1A2, 2C9, 2C19, 2D6 and 3A4 at pharmacologically relevant concentrations. | |
| ● | Better treatment option. As an orally administered treatment tested to work in as little as 1 hour, ANEB-001 has the potential to be faster acting than intravenous (IV) treatments that may be developed by competitors. ANEB-001 has the potential to be the first treatment of its kind as there are no other treatments approved by the FDA currently available to specifically reverse the symptoms of cannabinoid overdose. | |
| ● | No serious adverse events. A single dose of the drug is unlikely to produce adverse events associated with chronic dosing. The most commonly reported adverse effect in our Phase 1 study was gastrointestinal discomfort, which also occurred in subjects who were administered a placebo. |
We plan to commence a Phase 2 proof - of - concept study in the fourth quarter of 2021 at a center in the Netherlands to test the efficacy of a single dose of ANEB-001 on a population of approximately 100 human subjects who have been administered 10 milligrams of THC that will then be randomized to receive a placebo, low dose, medium dose or high dose of ANEB-001. We anticipate completing the Phase 2 study within approximately six months after commencing the study and having data potentially available in the first half of 2022. We believe this study will lay the foundation for us to engage with the FDA and/or comparable foreign regulatory authorities, file an Investigational New Drug Application (“IND”) with the FDA in the United States and conduct more extensive clinical trials with the goal of generating additional clinical data that will ultimately enable us to file a marketing application with the FDA.
We have engaged contract research organizations (“CROs”) to assist us with conducting clinical trials and to provide us with consulting and development services in the various phases of the drug development process. We currently have a consultancy agreement with Traxeus Pharma Services Limited (“Traxeus”), pursuant to which Traxeus provides certain pharmaceutical development services and deliverables to us, including manufacturing and testing a demonstration batch of the drug substance and completing the formulation and process development for the drug product. We plan to continue to engage CROs like Traxeus and other pharmaceutical services providers to assist us with clinical trials, the development of our lead product candidate ANEB-001.
| 8 |
Exclusive Worldwide License Agreement
On May 26, 2020, we entered into an exclusive license agreement (the “License Agreement”) with Vernalis. Pursuant to the License Agreement, Vernalis granted us an exclusive worldwide royalty-bearing license to develop and commercialize a compound that we refer to as ANEB-001. In exchange for the exclusive license, we agreed to pay Vernalis a non-refundable signature fee, certain developmental milestone and sales milestone payments subject to maximum caps, and low to mid-single digit royalties on net sales.
Under the License Agreement, we have the sole discretion to carry out the development and commercialization of ANEB-001, including obtaining regulatory approvals. We have access to certain regulatory materials, including study reports from clinical and non-clinical trials. We retain the sole right over certain patent rights (including patent applications) and know-how controlled by us that are necessary or reasonably useful to developing and commercializing ANEB-001 during the term of the License Agreement.
The License Agreement continues for an indefinite term and terminates, among other ways, under the following circumstances: (i) on its terms when royalties and other sums cease to be payable thereunder; (ii) by us at any time by providing 60 days’ prior notice; or (iii) upon an event of default, such as a material breach or insolvency of the other party. Upon termination, all rights and licenses granted by Vernalis will revert immediately to Vernalis; all outstanding sums as of the termination date will be immediately due and payable to Vernalis; and we will return or destroy, at Vernalis’s request, any regulatory or other materials provided by Vernalis pursuant to the License Agreement. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Private Placement and Recapitalization
On June 18, 2020, we received gross proceeds of $3.0 million from a private placement of our series A preferred stock (the “Private Placement”), convertible into 341,250 shares of our common stock, pursuant to the terms of a securities purchase agreement (the “Securities Purchase Agreement”) with 22NW, LP, an institutional accredited investor affiliated with Aron R. English, who became a director of our company at such time. The series A preferred stock is convertible into shares of common stock automatically upon the closing of this offering. The conversion price of the series A preferred stock is $8.7912 per share. The conversion price is subject to adjustment if, at any time prior to conversion of the shares, we issue in a financing additional shares of common stock or other equity or equity-linked securities at a purchase, conversion or exercise price less than $8.7912 per share. In any such case, we have agreed to issue additional shares of series A preferred stock to the investors so that the effective purchase price per share in the Private Placement is reduced by a weighted-average anti-dilution percentage that takes into account both the lower per share purchase, conversion or exercise price and the number of such additional shares issued at the lower price. No adjustment will be made, however, in respect of shares of common stock or stock options issued to employees, directors or consultants, or in connection with acquisitions of other corporations or strategic collaborations approved by our board of directors.
As part of the Private Placement, 22NW, LP and Mr. English, individually, further agreed under the Securities Purchase Agreement to purchase upon the achievement of certain corporate events “milestone” warrants for $1.95754 per warrant (or $2,250,000 in the aggregate). The warrants are exercisable for cash for up to 1,149,401 shares of series A preferred stock at an exercise price of $10.11 per share or on a “net-exercise” basis into such lesser number of shares of series A preferred stock by surrendering a portion of the underlying warrant shares, based on the positive difference between the stated warrant exercise price and the initial public offering price per share in this offering, to pay the exercise price. The warrants must be purchased upon our achievement of (i) a filing with the FDA of an investigational new drug application or the making of an analogous regulatory filing in any foreign jurisdiction, whichever is earlier, and (ii) an arrangement by us to produce the API of ANEB-001 in amounts sufficient to facilitate the consummation of a trial pursuant to such regulatory filing. The milestone warrants may also be purchased at any time at the option of 22NW, LP and Mr. English.
The financial statements of our company in this prospectus do not reflect the conversion of our series A preferred stock or the sale of our milestone warrants. The effect of the transactions on the financial statements will be to increase our outstanding shares of common stock as a result of the conversion of all our outstanding series A preferred stock into shares of common stock automatically upon the closing of this offering and increase our cash by $2,250,000 from the proceeds of the sale of our milestone warrants, in each case, before giving effect to the closing of this offering. See “Capitalization,” “Dilution” and “Certain Relationships and Related Transactions.”
| 9 |
Selected Risks Associated with Our Business
Investing in our common stock involves a high degree of risk. You should carefully consider all the information in this prospectus prior to investing in our common stock. These risks are discussed more fully in the section entitled “Risk Factors” immediately following this prospectus summary. Below are the principal factors that make an investment in our company speculative or risky:
| ● | we are a biotechnology company with a limited operating history; | |
| ● | we expect to incur significant losses and may never become profitable or be able to sustain profitability; | |
| ● | the proceeds of this offering will only fund our operations for a limited time and we will need to raise additional capital to support our development and commercialization efforts; | |
| ● | we have only one product candidate in process and are not currently focused on acquiring additional product candidates in the future; | |
| ● | even if we receive regulatory approval for ANEB-001, we may not be able to successfully commercialize this product and the revenue that we generate from sales, if any, may be limited; | |
| ● | our current pipeline program and future product candidates may not be successful; | |
| ● | clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; | |
| ● | failure to perform by third parties in the development and commercialization of our product candidates, including contract research organizations (“CROs”) and contract manufacturing organizations (“CMOs”), could delay our ability to receive regulatory approval; | |
| ● | although we may pursue an expedited regulatory approval pathway for ANEB-001, this product candidate may not qualify for expedited development, or if it does qualify for expedited development, it may not actually lead to a faster development or regulatory review or approval process; | |
| ● | our reliance on a license from a third party in relation to our rights and development of ANEB-001; | |
| ● | we depend on rights to certain pharmaceutical compounds that have been licensed to us and we do not control these pharmaceutical compounds and any loss of our rights to them could prevent us from selling our product if it is approved for marketing; | |
| ● | we have two pending patent applications and no issued or granted patents covering ANEB-001 or other product candidates; | |
| ● | if product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidate; and | |
| ● | the continuing novel coronavirus pandemic (Covid-19) could adversely affect our business, as well as the businesses of the CROs and CMOs we engage to assist in the development and commercialization of our product candidates. |
Implications of Being an “Emerging Growth Company”
As a company with less than $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” under the Jumpstart our Business Startups Act of 2012 (the “JOBS Act”). An emerging growth company may take advantage of certain reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. In particular, as an emerging growth company we:
| ● | are not required to obtain an attestation and report from our auditors on our management’s assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act; |
| 10 |
| ● | are not required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion and analysis”); | |
| ● | are not required to obtain a non-binding advisory vote from our stockholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute” votes); | |
| ● | are exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; | |
| ● | may present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis of Financial Condition and Results of Operations, or MD&A; and | |
| ● | are eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. |
We intend to take advantage of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under §107 of the JOBS Act. Please see “Risk Factors – “We are an ‘emerging growth company.’”
Certain of these reduced reporting requirements and exemptions were already available to us due to the fact that we also qualify as a “smaller reporting company” under the Securities and Exchange Commission (“SEC”) rules. For instance, smaller reporting companies are not required to obtain an auditor attestation and report regarding internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act of 1933, as amended, or such earlier time that we no longer meet the definition of an emerging growth company. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1.07 billion in annual revenue, have more than $700 million in market value of our common stock held by non-affiliates, or issue more than $1 billion in principal amount of non-convertible debt over a three-year period. Further, under current SEC rules we will continue to qualify as a “smaller reporting company” for so long as we have a public float (i.e., the market value of common equity held by non-affiliates) of less than $250 million as of the last business day of our most recently completed second fiscal quarter.
Corporate Information and Incorporation
Anebulo Pharmaceuticals, Inc. was incorporated in the State of Delaware on April 23, 2020. Our principal executive offices are located at 1415 Ranch Road 620 South, Suite 201, Lakeway, Texas 78734, and our telephone number is (512) 598-0931. You may access our website at www.anebulo.com. Our website and the information contained therein or connected thereto shall not be deemed to be incorporated into this prospectus or the registration statement of which it forms a part.
| 11 |
THE OFFERING
The summary below describes the principal terms of this offering. The “Description of Capital Stock” section of this prospectus contains a more detailed description of our common stock.
| Common stock offered by us | shares. | |
| Proposed initial public offering price | $ per share. | |
| Underwriters’ over-allotment option | We have granted the underwriters an option for a period of 30 days from the date of this prospectus to purchase up to an additional shares of our common stock from us at the initial public offering price less underwriting discounts and commissions to cover over-allotments, if any. | |
| Common stock to be outstanding immediately after this offering | shares (or shares if the underwriters’ option to purchase additional shares of our common stock from us is exercised in full).(1) | |
| Use of proceeds | We estimate that the net proceeds from the sale of shares of our common stock in this offering will be approximately $ million (or approximately $ million if the underwriters option to purchase additional shares of our common stock from us is exercised in full), based on the assumed initial public offering price of $ per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. | |
| We intend to use the net proceeds of this offering to make expenditures to fund proprietary research and development of our ANEB-001 product candidate and to support preclinical testing and clinical trials necessary for regulatory filings. A portion of the net proceeds of this offering may be used for the acquisition or licensing of complementary technologies, products or businesses. The net proceeds of this offering will also be available for working capital and other general corporate purposes, including enhancing our corporate infrastructure and systems to assist in creating a more robust means of tracking data, automating back office functions and improving our financial reporting system. See “Use of Proceeds.” | ||
| Dividend policy | We have never declared or paid any cash dividends on our common stock. We anticipate that we will retain any earnings to support operations and to finance the growth and development of our business. Accordingly, we do not expect to pay cash dividends on our common stock in the foreseeable future. | |
| Risk factors | Investing in our common stock involves a high degree of risk. See “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in shares of our common stock. | |
| Proposed listing | We intend to apply to list our common stock on The Nasdaq Capital Market in connection with this offering. | |
| Proposed Nasdaq trading symbol | “ANEB”(2) |
| (1) | In this prospectus, except as otherwise indicated, the number of shares of our common stock that will be outstanding immediately after this offering and the other information based thereon: | |
| ● | assumes an initial public offering price of $ per share of common stock, which is the midpoint of the estimated public offering price range set forth on the cover page of this prospectus; | |
| ● | assumes the sale of our milestone warrants and the conversion of all our series A preferred stock into shares of common stock automatically upon the closing of this offering (see “Prospectus Summary – Private Placement and Recapitalization”); | |
| ● | excludes _____ shares of common stock reserved for issuance upon the exercise of outstanding stock options awarded to our non-employee directors in 2021, and _____ shares of common stock reserved for future issuance under our 2020 Stock Incentive Plan. | |
| ● | no exercise by the underwriters of their option to purchase up to an additional shares of our common stock from us in this offering to cover over-allotments, if any. | |
| (2) | We have reserved the trading symbol “ANEB” in connection with our application to have our common stock listed for trading on The Nasdaq Capital Market. | |
| 12 |
SUMMARY FINANCIAL DATA
The following tables present our summary financial data. We derived the summary statement of operations data for the period from April 23, 2020 (inception) to June 30, 2020 and the balance sheet data as of June 30, 2020 from our audited financial statements included elsewhere in this prospectus. The summary statements of operations for the six months ended December 31, 2020 and the summary balance sheet data as of December 31, 2020 are derived from our interim financial statements included elsewhere in this prospectus. We have prepared the interim financial statements on the same basis as the audited financial statements and have included, in our opinion, all adjustments consisting only of normal recurring adjustments that we consider necessary for a fair statement of the financial information set forth in those statements. You should read the following summary financial data in conjunction with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements, related notes and other financial information included elsewhere in this prospectus. The summary financial data in this section is not intended to replace the financial statements included elsewhere in this prospectus and is qualified in its entirety by the financial statements, related notes and other financial information included elsewhere in this prospectus. Our historical results for any prior period are not necessarily indicative of our future results, and our operating results for the period from April 23, 2020 (inception) to June 30, 2020 and six months ended December 31, 2020 are not necessarily indicative of the results that may be expected for the fiscal year ending June 30, 2021 or any other interim periods or any future year or period.
| Six Months ended December 31, 2020 | For
the period from April 23, 2020 (inception) | |||||||
| Statement of Operations Data: | ||||||||
| Operating expenses: | ||||||||
| Research and development | $ | 190,268 | $ | 150,000 | ||||
| General and administrative | 386,649 | 23,351 | ||||||
| Total operating expenses | 576,917 | 173,351 | ||||||
| Other expense: | ||||||||
| Interest expense | (8,066 | ) | (1,286 | ) | ||||
| Loss before provision for income taxes | (584,983 | ) | (174,637 | ) | ||||
| Income tax expense | - | - | ||||||
| Net loss | $ | (584,983 | ) | $ | (174,637 | ) | ||
| Weighted average common shares outstanding, basic and diluted | 2,136,162 | 2,000,000 | ||||||
| Net loss per share, basic and diluted | $ | (0.27 | ) | $ | (0.09 | ) | ||
| December 31, 2020 | ||||||||||||
| Actual(1) | Pro Forma(2) | Pro Forma, as Adjusted(3) | ||||||||||
| (unaudited) | (unaudited) | |||||||||||
| Balance Sheet Data: | ||||||||||||
| Cash and cash equivalents | $ | 2,480,003 | $ | $ | ||||||||
| Working capital | 2,155,083 | |||||||||||
| Total assets | 2,610,509 | |||||||||||
| Convertible preferred stock | 2,975,752 | |||||||||||
| Total stockholders’ equity (deficit) | (719,018 | ) | ||||||||||
| (1) | Actual balance sheet data presents balance sheet data on an actual basis without any adjustments to reflect subsequent or anticipated events. |
| (2) | Pro forma balance sheet data presents balance sheet data on a pro forma basis reflecting the receipt by us of the proceeds from the sale of our milestone warrants for a total of $2,250,000 before the closing of this offering, and the conversion of all our series A preferred stock into shares of common stock automatically upon the closing of this offering (the “Recapitalization”). See “Capitalization” and “Certain Relationships and Related Transactions.” |
| (3) | Pro forma, as adjusted balance sheet data presents balance sheet data on a pro forma, as adjusted basis reflecting the Recapitalization and the receipt by us of the net proceeds from the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the estimated offering price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and excluding the exercise of the underwriters’ over-allotment option, as if each had occurred on December 31, 2020. See “Use of Proceeds.” |
| 13 |
An investment in our common stock involves a high degree of risk. Prior to making a decision about investing in our common stock, you should carefully consider the risks and uncertainties described below, together with all of the other information in this prospectus, including the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes. The risks and uncertainties we have described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe are not material may also affect our business, results of operations, financial condition or prospects. The occurrence of any of these known or unknown risks might cause the market price of our common stock to decline, and might cause you to lose all or part of your investment.
Risks Related to our Business, Financial Condition and Capital Requirements
We have not generated any revenue since our inception and expect to incur future losses and may never become profitable.
We have not generated any revenue. As of December 31, 2020, we have an accumulated deficit of $759,620. The likelihood of our future success must be considered in light of the expenses, difficulties, complications and delays often encountered in connection with the clinical trials that will be conducted and on the development of new solutions to common addictions. These potential challenges include, but are not limited to, unanticipated clinical trial delays, poor data, changes in the regulatory and competitive landscape and additional costs and expenses that may exceed current budget estimates. In order to complete certain clinical trials and otherwise operate pursuant to our current business strategy, we anticipate that we will incur increased operating expenses. In addition, we expect to incur significant losses and experience negative cash flow for the foreseeable future as we fund the operating losses and capital expenditures. We recognize that if we are unable to generate sufficient revenues or source funding, we will not be able to continue operations as currently contemplated, complete planned clinical trials and/or achieve profitability. Our failure to achieve or maintain profitability will also negatively impact the value of our shares. If we are unsuccessful in addressing these risks, then we may need to curtail our business activities.
The future success of our business cannot be determined at this time, and we do not anticipate generating revenue from product sales for the foreseeable future. In addition, we have no experience in commercializing drug products on our own and face a number of challenges with respect to commercialization efforts, including, among other challenges:
| ● | having inadequate financial or other resources to complete the development of our product candidate; | |
| ● | the inability to manufacture our product in commercial quantities, at an adequate quality, at an acceptable cost or in collaboration with third parties; | |
| ● | experiencing delays or unplanned expenditures in product development, clinical testing or manufacturing; | |
| ● | the inability to establish adequate sales, marketing and distribution channels; | |
| ● | healthcare professionals may not adopt and patients may not accept our drug, if approved for marketing; | |
| ● | we may not be aware of possible complications or other side effects from the use of our product since we have limited clinical experience with respect to the actual effects from use of our product; | |
| ● | technological breakthroughs in reversing cannabinoid overdoses and treating patients experiencing overdose symptoms may reduce the demand for our product, if it develops; | |
| ● | changes in the market for reversing cannabinoid overdoses and treating patients experiencing overdose symptoms, new alliances between existing market participants and the entrance of new market participants may interfere with our market penetration efforts; | |
| ● | third-party payors may not agree to reimburse patients for any or all of the purchase price of our product, which may adversely affect patients’ willingness to use our product; |
| 14 |
| ● | uncertainty as to market demand may result in inefficient pricing of our product; | |
| ● | we may face third-party claims of intellectual property infringement; | |
| ● | we may fail to obtain or maintain regulatory approvals for our product in our markets or may face adverse regulatory or legal actions relating to our product even if regulatory approval is obtained; and | |
| ● | we are dependent upon the results of clinical studies relating to our product and the products of our competitors. If data from a clinical trial is unfavorable, we would be reluctant to advance the product for the indication for which it was being developed. |
If we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our products could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
We currently rely on a license from a third party, and in the future may rely on additional licenses from other third parties, in relation to our development of ANEB-001, and if we fail to comply with our obligations under our current or future intellectual property license agreements or otherwise experience disruptions to our business relationships with our current or any future licensors, we could lose intellectual property rights that are important to our business.
We are, and expect to continue to be, reliant upon third-party licensors for certain patent and other intellectual property rights that are important or necessary to the development of our product candidates, including ANEB-001. On May 26, 2020, we entered into the License Agreement with Vernalis, pursuant to which Vernalis granted to us an exclusive license to develop and commercialize our ANEB-001 product candidate. Under the License Agreement, we have the sole discretion to carry out the development and commercialization of ANEB-001, including obtaining regulatory approvals. We retain the sole right over certain patent rights (including patent applications) and know-how controlled by us that are necessary or reasonably useful to developing and commercializing the licensed product during the term of the License Agreement. The License Agreement imposes, and we expect that any future license agreement will impose, specified diligence, milestone payment, royalty, commercialization, development and other obligations on us and require us to meet development timelines, or to exercise diligent or commercially reasonable efforts to develop and commercialize licensed products, in order to maintain the license.
Furthermore, our licensors have, or may have in the future, the right to terminate a license if we materially breach the agreement and fail to cure such breach within a specified period or in the event we undergo certain bankruptcy events. In spite of our best efforts, our current or any future licensors might conclude that we have materially breached our license agreements and might therefore terminate the license agreements. If our license agreements are terminated, we may lose our rights to develop and commercialize product candidates and technology, lose patent protection, experience significant delays in the development and commercialization of our product candidates and technology, and incur liability for damages. If these in-licenses are terminated, or if the underlying intellectual property fails to provide the intended exclusivity, our competitors or other third parties could have the freedom to seek regulatory approval of, and to market, products and technologies identical or competitive to ours and we may be required to cease our development and commercialization of certain of our product candidates and technology. In addition, we may seek to obtain additional licenses from our licensors and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensors, including by agreeing to terms that could enable third parties, including our competitors, to receive licenses to a portion of the intellectual property that is subject to our existing licenses and to compete with any product candidates we may develop and our technology. Any of the foregoing could have a material adverse effect on our competitive position, business, financial condition, results of operations and prospects.
Our License Agreement with Vernalis continues for an indefinite term and terminates, among other ways, under the following circumstances: (i) on its terms when royalties and other sums cease to be payable thereunder; (ii) by us at any time by providing 60 days’ prior notice; or (iii) upon an event of default, such as a material breach or insolvency of the other party. Upon termination, all rights and licenses granted by Vernalis will revert immediately to Vernalis; all outstanding sums as of the termination date will be immediately due and payable to Vernalis; and we will return or destroy, at Vernalis’s request, any regulatory or other materials provided by Vernalis pursuant to the License Agreement.
| 15 |
Disputes may also arise between us and Vernalis or future licensors regarding intellectual property subject to a license agreement, including:
| ● | the scope of rights granted under the license agreement and other interpretation-related issues; | |
| ● | our financial or other obligations under the license agreement; | |
| ● | whether, and the extent to which, our products, technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; | |
| ● | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; | |
| ● | the inventorship and ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensor(s); and | |
| ● | the priority of invention of patented technology. |
If we do not prevail in such disputes, we may lose any or all of our rights under such license agreements, experience significant delays in the development and commercialization of our products and technologies, or incur liability for damages, any of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. In addition, we may seek to obtain additional licenses from our licensor(s) and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensor(s), including by agreeing to terms that could enable third parties, including our competitors, to receive licenses to a portion of the intellectual property that is subject to our existing licenses and to compete with our products.
In addition, the agreements under which we currently and in the future license intellectual property or technology from third parties are complex and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology, or increase what we believe to be our financial or other obligations under the relevant agreement, either of which could have a material adverse effect on our business, financial condition, results of operations and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize any affected products or services, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Absent the license agreements, we may infringe patents subject to those agreements, and if the license agreements are terminated, we may be subject to litigation by the licensor. Litigation could result in substantial costs to us and distract our management. If we do not prevail, we may be required to pay damages, including treble damages, attorneys’ fees, costs and expenses and royalties or be enjoined from selling ANEB-001, which could adversely affect our ability to offer products or services, our ability to continue operations and our business, financial condition, results of operations and prospects.
We currently have no product revenue and will need to raise additional capital following this offering, which may be unavailable to us or may cause dilution or place significant restrictions on our ability to operate.
For the foreseeable future, we may be unable to generate sufficient revenue or cash flow to fund our operations. We will need to seek additional equity or debt financing following this offering to provide the capital required to maintain or expand our operations, continue the development of our product candidate, build our sales and marketing capabilities, promote brand identity, develop or acquire complementary technologies, products or businesses, or provide for our working capital requirements and other operating and general corporate purposes.
Other than this offering, we do not have any other arrangements or credit facilities as a source of funds, and we make no assurance that we will be able to raise sufficient additional capital in the future if needed on acceptable terms, or at all. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back or eliminate the development of our current product or future candidates and other business. This may materially adversely affect our operations and financial condition as well as our ability to achieve business objectives and maintain competitiveness. Our inability to fund our business could thus lead to the loss of your investment.
| 16 |
If we raise additional capital by issuing equity securities and/or equity-linked securities, the percentage ownership of our existing stockholders may be reduced, and accordingly these stockholders may experience substantial dilution. We may also issue equity securities and/or equity-linked securities that provide for rights, preferences and privileges senior to those of our common stock. Given our need for cash and that equity and equity-linked issuances are very common types of fundraising for companies like us, the risk of dilution is particularly significant for our stockholders.
Debt financing, if obtained, may involve agreements that include liens on our assets and covenants limiting or restricting our ability to take specific actions such as incurring additional debt. Debt financing could also be required to be repaid regardless of our operating results.
If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our current or future products or to grant licenses on terms that are not favorable to us.
We have no operating history as a publicly-traded company, and our inexperience could materially and adversely affect us and our stockholders.
We have no operating history as a publicly-traded company. Our board of directors and management team will have overall responsibility for our management. As a publicly-traded company, we will be required to develop and implement substantial control systems, policies and procedures in order to satisfy our periodic SEC reporting and Nasdaq obligations. We cannot assure you that management’s past experience will be sufficient to successfully develop and implement these systems, policies and procedures and to operate our company. Failure to do so could jeopardize our status as a public company, and the loss of such status may materially and adversely affect us and our stockholders.
We depend on third parties in connection with our preclinical testing and clinical trials, which may result in costs and delays that prevent us from obtaining regulatory approval or successfully commercializing ANEB-001 or future product candidates.
We engage third parties to perform various aspects of our preclinical testing and clinical trials. We have entered into agreements with third parties, including Traxeus, Aptuit (Verona) SRL, and Centre for Human Drug Research, which provide certain pharmaceutical research and development services to us. For more information regarding our contracts with these third parties, see “Business—Our Clinical Trials and Milestones.” We depend on these third parties to perform these activities on a timely basis in accordance with the protocol, good laboratory practices, good clinical practices and other regulatory requirements. Our reliance on these third parties for preclinical and clinical development activities reduces our control over these activities. Accordingly, if these parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, our preclinical testing and clinical trials may be extended, delayed, terminated or our data may be rejected by the FDA. If there are delays in testing or obtaining regulatory approvals as a result of a third party’s failure to perform, our drug discovery and development costs will likely increase, and we may not be able to obtain regulatory approval for or successfully commercialize our current or future product candidates.
Third parties’ abilities to adequately and timely manufacture and supply our current or future product candidates is dependent on the operation of their facilities which may be impacted by, among other things:
| ● | availability, performance or contamination of raw materials and components used in the manufacturing process, particularly those for which we have no other source or supplier; | |
| ● | capacity of their facilities; | |
| ● | the performance of information technology systems; | |
| ● | compliance with regulatory requirements; | |
| ● | inclement weather and natural disasters; | |
| ● | changes in forecasts of future demand for product components; | |
| ● | timing and actual number of production runs for product components; |
| 17 |
| ● | potential facility contamination by microorganisms or viruses; | |
| ● | updating of manufacturing specifications; and | |
| ● | product quality success rates and yields. |
If the efficient manufacture and supply of our current or future product candidates is interrupted, we may experience delayed shipments or supply constraints, which may materially impact our ongoing and future preclinical testing and clinical trials.
Any contract manufacturer must undergo a potentially lengthy FDA approval process, as well as other regulatory approval processes, and are subject to continued review by the FDA and other regulatory authorities. If we or our third-party service providers cease or interrupt production or if our third-party service providers fail to supply materials, products or services to us, we may experience delayed shipments, and supply constraints for our current or future product candidates.
Public health epidemics, pandemics or outbreaks, including the recent novel coronavirus pandemic (Covid-19), could adversely affect our business.
In December 2019, the novel coronavirus (“Covid-19”) was identified in Wuhan, China. The virus continues to spread globally, has been declared a pandemic by the World Health Organization and has spread to over 100 countries, including the United States. The Covid-19 outbreak is significantly affecting our communities, our business operations and the business operations of the CROs and CMOs we have engaged, as well as the U.S. economy and financial markets. The full extent to which the Covid-19 outbreak will impact our business, results of operations, financial condition and cash flows will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning Covid-19 and the actions to contain it or treat its impact and the economic impact on local, regional, national and international markets. As the Covid-19 pandemic continues, our results of operations, financial condition and cash flows are likely to be materially adversely affected, particularly if the pandemic persists for a significant amount of time.
Covid-19 or other public health epidemics, pandemics or outbreaks, and the resulting business or economic disruptions resulting therefrom, may adversely impact our business as well as our ability to raise capital. The impact of this pandemic has been and will likely continue to be extensive in many aspects of society, which has resulted in and will likely continue to result in significant disruptions to the global economy, as well as businesses and capital markets around the world.
While we cannot presently predict the scope and severity of any potential business shutdowns or disruptions, if we or any of our business partners, clinical trial sites, distributors and other third parties with whom we conduct business, were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted. For example, if our development program for cannabinoid overdoses were to be delayed, it may have a material adverse effect on our business, results of operations and financial condition.
The pandemic’s impact on the medical community and the global economy could have an adverse impact on future sales upon which we expect to derive royalties and milestones, which could lead to a decrease in our revenues, net income and assets.
Several measures have been and are currently being implemented by the United States and other governments to address the current Covid-19 pandemic and its economic impacts. At this time, it is impossible to predict the success of these measures and whether or not they will have unforeseen negative consequences for our business. In addition, our results of operations, financial position and cash flows may be adversely affected by federal or state laws, regulations, orders, or other governmental or regulatory actions addressing the current Covid-19 pandemic or the U.S. healthcare system, which, if adopted, could result in direct or indirect restrictions to our business, results of operations, financial condition and cash flow.
The foregoing and other continued disruptions to our business as a result of Covid-19 could result in a material adverse effect on our business, results of operations, financial condition and cash flows. Further, the Covid-19 pandemic could heighten the risks in certain of the other risk factors described herein.
| 18 |
Our current and future operations substantially depend on our Founder and Chief Executive Officer and our ability to hire other key personnel, the loss of any of whom could disrupt our business operations.
Our business depends and will continue to depend in substantial part on the continued service of Joseph F. Lawler, M.D., Ph.D., our founder and a director, and Daniel Schneeberger, M.D., our Chief Executive Officer and a director. The loss of the services of Dr. Lawler or Dr. Schneeberger would significantly impede implementation and execution of our business strategy and may result in the failure to reach our goals. Further, the loss of either Dr. Lawler or Dr. Schneeberger would be negatively perceived in the capital markets. We do not have “key-man” life insurance for our benefit on the lives of either Dr. Lawler or Dr. Schneeberger.
Our future viability and ability to achieve sales and profits will also depend on our ability to attract, train, retain and motivate highly qualified personnel in the diverse areas required for continuing operations. There is a risk that we will be unable to attract, train, retain or motivate qualified personnel, both near term or in the future, and the failure to do so may severely damage our prospects.
Our employment agreement with our Chief Executive Officer may require us to pay severance benefits to him if terminated in connection with a change in control of us which could harm our financial condition or results.
We have entered into an employment agreement with Dr. Schneeberger to serve as our Chief Executive Officer. The employment agreement contains change in control and severance provisions. In the event of a change in control of our company, Dr. Schneeberger will be entitled to the vesting of 50% of any stock-based awards granted but not yet vested prior to the change in control event not less than six months after the change in control event, provided Dr. Schneeberger remains employed by our company. If the change in control event is an initial public offering, Dr. Schneeberger will be entitled to the full vesting of any stock-based awards. In the event of Dr. Schneeberger’s termination, Dr. Schneeberger will be entitled to severance payments as follows: (i) if terminated by us without cause or upon his resignation for good reason, severance payments will be equal to the remainder of the annual base compensation for the year in which the date of termination occurs and the immediate award and vesting of the next quarterly stock-based award; and (ii) if terminated due to non-extension of the initial term, and only if we exercise our non-compete option, severance payments will be equal to the annual base compensation for the year in which the date of termination occurs, multiplied by a fraction, the numerator of which is equal to the number of days from the date of termination through the one-year anniversary thereof and the denominator of which is 365. The accelerated vesting of options and restricted stock units could result in dilution to our existing stockholders and harm the market price of our common stock. The payment of these severance benefits could harm our financial condition and results. In addition, these potential severance payments may discourage or prevent third parties from seeking a business combination with us.
Risks Related to our Intellectual Property
If we are unable to obtain and maintain patent protection for important aspects of ANEB-001, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize products that are similar or identical to ours, and our ability to successfully commercialize our current or future product candidates may be adversely affected.
Our commercial success will depend, in part, on our ability to obtain and maintain patent protection in the United States and other countries with respect to ANEB-001, our product candidate. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to aspects of our product candidate that are important to our business. Given that the development of our product candidates is at an early stage, our intellectual property portfolio with respect to certain aspects of our product candidates is also at an early stage. For example, we have filed or intend to file patent applications related to aspects of ANEB-001, our product candidate; however, there can be no assurance that any such patent applications will issue as granted patents around the world. The requirements for patentability differ in certain countries, and certain countries have heightened requirements for patentability. Further, in some cases, we have only filed provisional patent applications on certain aspects of our technology and product candidate, and provisional patent applications are not eligible to become an issued patent until, among other things, we file a non-provisional patent application within 12 months of the filing date of the applicable provisional patent application. Any failure to file a non-provisional patent application within this timeline could cause us to lose the ability to obtain patent protection for the inventions disclosed in the associated provisional patent applications.
Further, any changes we make to our product candidates to cause them to have what we view as more advantageous properties may not be covered by our existing patent applications, and we may be required to file new applications and/or seek other forms of protection for any such altered product candidates. There can be no assurance that we would be able to secure patent protection that would adequately cover any such altered product candidates. There can also be no assurance that any such patent applications will be issued as granted patents, and even if they do issue, such patent claims may be insufficient to prevent third parties, such as our competitors, from utilizing our technology. Any failure to obtain or maintain patent protection related to aspects of our product candidates could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Even if we obtain issued or granted patents with respect to our product candidates, we cannot be certain that such patents will not later be found to be invalid and/or unenforceable. Currently, we do not have patents on our core intellectual property.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Although we may enter into non-disclosure and confidentiality agreements with parties who have access to patentable aspects of our research and development output, such as our employees, distribution partners, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection.
| 19 |
The patent position of pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our potential patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued, and even if issued, the patents may not meaningfully protect our current or future product candidates, effectively prevent competitors and third parties from commercializing competitive products or otherwise provide us with any competitive advantage. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative products in a non-infringing manner.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Patent applications we own currently or that in the future issue as patents may not be issued in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. Any patents to which we have rights may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, we do not know whether our product candidates will be protectable or remain protected by valid and enforceable patents.
The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. We may be subject to a third-party pre-issuance submission of prior art to the United States Patent and Trademark Office (the “USPTO”) or post-issuance become involved in opposition, derivation, revocation, reexamination, post-grant and inter partes review, or interference proceedings or other similar proceedings challenging our patent rights. An adverse determination in any such submission, proceeding, or litigation could reduce the scope of, or invalidate or render unenforceable, such patent rights, allow third parties to commercialize our product candidates or other technologies and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Moreover, we may have to participate in interference proceedings declared by the USPTO to determine priority of invention or in post-grant challenge proceedings, such as post-grant review at the USPTO or oppositions in a foreign patent office, that challenge our priority of invention or other features of patentability with respect to our patents and patent applications. Such challenges may result in loss of patent rights, loss of exclusivity, or in patent claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our product candidates and other technologies. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
If we are unsuccessful in any such proceeding or other priority or inventorship dispute, we may be required to obtain licenses from third parties, including parties involved in any such interference proceedings or other priority or inventorship disputes. Such licenses may not be available on commercially reasonable terms or at all, or may be non-exclusive. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture, and commercialization of one or more of the product candidates we may develop. Termination of these licenses or reduction or elimination of our rights under these licenses may result in our having to negotiate new or reinstated agreements with less favorable terms, or cause us to lose our rights under these licenses, including our rights to important intellectual property or technology. The loss of exclusivity or the narrowing of our owned and licensed patent claims could limit our ability to stop others from using or commercializing similar or identical technology and products.
In addition, given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such product candidates might expire before or shortly after such product candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Some of our patents and patent applications may in the future be co-owned with third parties. In addition, future collaborators or licensors may co-own their patents and patent applications with other third parties with whom we do not have a direct relationship. Our rights to certain of these patents and patent applications may be dependent, in part, on inter-institutional or other operating agreements between the joint owners of such patents and patent applications, who are not parties to our license agreements. If our future collaborators or licensors do not have exclusive control of the grant of licenses under any such third-party co-owners’ interest in such patents or patent applications or we are otherwise unable to secure such exclusive rights, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology to the extent such products and technology are not also covered by our intellectual property. In addition, we may need the cooperation of any such co-owners of our patents in order to enforce such patents against third parties, and such cooperation may not be provided to us.
We cannot be certain that our potential patent rights will be effective in protecting ANEB-001 and related technologies. Failure to protect such assets may have a material adverse effect on our business, operations, financial condition and prospects.
If we do not obtain patent term extension and data exclusivity for any product candidates we may develop, our business may be materially harmed.
Depending upon the timing, duration, and specifics of any FDA marketing approval of ANEB-001 and related technologies we may develop, one or more of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Act). The Hatch-Waxman Act permits a patent term extension of up to five years as compensation for patent term lost during the FDA regulatory review process. A patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar extensions as compensation for patent term lost during regulatory review processes are also available in certain foreign countries and territories, such as in Europe under a Supplementary Patent Certificate. However, we may not be granted an extension in the United States and/or foreign countries and territories because of, for example, failing to exercise due diligence during the testing phase or regulatory review process, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is shorter than what we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations, and growth prospects could be materially harmed.
| 20 |
We may face litigation from third parties claiming that our product or business infringes, misappropriates or otherwise violates their intellectual property rights, or seeking to challenge the validity of our patent rights.
Our future success is also dependent in part on the strength of our intellectual property, trade secrets and know-how, and on our ability, and the ability of our future collaborators, to develop, manufacture, market and sell ANEB-001, if approved, and use our proprietary technologies without alleged or actual infringement, misappropriation or other violation of the patents and other intellectual property rights of third parties. Moreover, it is difficult to conclusively assess our freedom to operate without infringing on third-party rights. We may be exposed to, or be threatened with, adversarial proceedings or additional future litigation by third parties regarding intellectual property rights with respect to our current and any future product candidates and technology.
There have been many lawsuits and other proceedings involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, as well as administrative proceedings, including patent infringement lawsuits, interferences, post-grant review, inter partes review, oppositions and reexamination proceedings before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidate. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidate may be subject to claims of infringement of the intellectual property rights of third parties. We may become party to, or threatened with, such actions in the future, regardless of their merit.
Numerous U.S. and foreign issued patents and pending patent applications owned by third parties exist in the pharmaceutical and biotechnology fields which may impact development of our product candidates. We cannot assure you that the product candidate we have developed, are developing or may develop in the future will not infringe existing or future patents owned by third parties. We may not be aware of patents that have already been issued and that a third-party, for example, a competitor in the fields in which we are developing product candidates might assert that their rights are infringed by our current or future product candidates, including claims to compositions, formulations, methods of manufacture or methods of use or treatment that cover our product candidate. It is also possible that patents owned by third parties of which we are aware, but which we do not believe are relevant to our product candidate, could be found to be infringed by our product candidate. In addition, because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidate may infringe.
Third parties may assert infringement claims against us based on patents that may be granted in the future including, our patent applications, regardless of their merit. There is a risk that third parties may choose to engage in litigation with us to enforce or to otherwise assert their patent rights or to obtain injunctive or other equitable relief against us. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, and the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license under the applicable patents, or until such patents expire or are finally determined to be invalid or unenforceable. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our compositions, formulations, or methods of treatment, prevention or use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtained a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms, or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. Some claimants may have substantially greater resources than we do and may be able to sustain the costs of complex intellectual property litigation to a greater degree and for longer periods of time than we could. In addition, patent holding companies that focus solely on extracting royalties and settlements by enforcing patent rights may target us. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or to enable the commercialization of our current or future product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In such an event, we would be unable to further practice our technologies or develop and commercialize our current or future product candidates, which could harm our business significantly.
Similarly, we or our licensors or collaborators may initiate proceedings or litigation against third parties, for instance, to challenge the validity or scope of intellectual property rights controlled by third parties. In order to successfully challenge the validity of any U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present clear and convincing evidence as to the invalidity of any such United States patent claim, there is no assurance that a court would invalidate the claims of any such United States patent.
| 21 |
Patent litigation and other proceedings may also absorb significant management time. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Engaging in litigation to defend against third parties alleging that we have infringed, misappropriated, or otherwise violated their patents or other intellectual property rights is very expensive, particularly for a company of our size, and time-consuming. Some of our competitors may be able to sustain the costs of litigation or administrative proceedings more effectively than we can because of greater financial resources. In the event of a successful claim of infringement against us, we may be enjoined from further developing or commercializing our infringing product candidate. In addition, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties, and/or redesign our infringing product candidate, which may be impossible or require substantial time and monetary expenditure. In that event, we would be unable to further develop and commercialize our product candidate, which could harm our business significantly. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings against us could impair our ability to compete in the marketplace. The occurrence of any of the foregoing could have a material adverse effect on our business, financial condition, results of operations, and growth prospects.
During the course of any patent or other intellectual property litigation or other proceeding, there could be public announcements of the results of hearings, rulings on motions, and other interim proceedings or developments and if securities analysts or investors regard these announcements as negative, the perceived value of our current or future product candidates or intellectual property could be diminished. Accordingly, the market price of our common stock may decline. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our business, ability to compete in the marketplace, financial condition, results of operations and growth prospects.
We may become involved in lawsuits to protect or enforce our patent rights or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe, misappropriate or otherwise violate our patent rights, trademarks, copyrights or other intellectual property, or those of our licensors. To counter infringement, misappropriation, unauthorized use or other violations, we may be required to file legal claims, which can be expensive and time consuming and divert the time and attention of our management and scientific personnel.
We may not be able to prevent, alone or with our licensees or any future licensors, infringement, misappropriation or other violations of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their patents. In addition, in a patent infringement proceeding, there is a risk that a court will decide that a patent of ours is invalid or unenforceable, in whole or in part, and that we do not have the right to stop the other party from using the invention at issue. There is also a risk that, even if the validity of such patents is upheld, the court will construe the patent’s claims narrowly or decide that we do not have the right to stop the other party from using the invention at issue on the grounds that our patents do not cover the invention. An adverse outcome in a litigation or proceeding involving our patents could limit our ability to assert our patents against those parties or other competitors, and may curtail or preclude our ability to exclude third parties from making and selling similar or competitive products. Any of these occurrences could adversely affect our competitive business position, business prospects and financial condition. Similarly, if we assert trademark infringement claims, a court may determine that the marks we have asserted are invalid or unenforceable, or that the party against whom we have asserted trademark infringement has superior rights to the marks in question. In this case, we could ultimately be forced to cease use of such trademarks.
In any infringement, misappropriation or other intellectual property litigation, any award of monetary damages we receive may not be commercially valuable. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during litigation. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims, which typically last for years before they are concluded. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings.
If we initiated legal proceedings against a third-party to enforce a patent covering our product candidates or other technologies, the defendant could counterclaim that such patent is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may raise claims challenging the validity or enforceability of our patents before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in the revocation of, cancellation of, or amendment to our patents in such a way that they no longer cover our product candidate or other technologies. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a third-party were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidate or other technologies. Such a loss of patent protection would have a material adverse impact on our business, financial condition, results of operations, and growth prospects.
We may not be able to protect our intellectual property rights throughout the world, which could negatively impact our business.
Filing, prosecuting and defending patent rights on important aspects of ANEB-001 in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Further, licensing partners may not prosecute patents in certain jurisdictions in which we may obtain commercial rights, thereby precluding the possibility of later obtaining patent protection in these countries. Consequently, we may not be able to prevent from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may develop their own products and may also export infringing products to territories where we may have patent protection, but enforcement is not as strong as that in the United States. These products may compete with ANEB-001, and our patent or other intellectual property rights may not be effective or sufficient to prevent them from competing.
| 22 |
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patent rights or marketing of competing products in violation of our proprietary rights generally. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Furthermore, while we intend to protect our intellectual property rights in our expected significant markets, we cannot ensure that we will be able to initiate or maintain similar efforts in all jurisdictions in which we may wish to market our current or future product candidates. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate, which may have an adverse effect on our ability to successfully commercialize our current or future product candidates in all of our expected significant foreign markets.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our future collaborators or licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected. Changes in patent law in the United States and other jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our products.
Changes in either the patent laws or interpretation of the patent laws in the United States or other jurisdictions could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming that other requirements for patentability are met, prior to March 16, 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. On or after March 16, 2013, under the Leahy-Smith America Invents Act (the America Invents Act) enacted on September 16, 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third-party was the first to invent the claimed invention. A third-party that files a patent application in the USPTO on or after March 16, 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third-party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we were the first to either (i) file any patent application related to ANEB-001 or (ii) invent any of the inventions claimed in our patents or patent applications.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third-party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third-party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third-party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third-party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biopharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
We may be subject to claims that we or our employees, consultants, contractors or advisors have infringed, misappropriated or otherwise violated the intellectual property of a third party, or claiming ownership of what we regard as our own intellectual property.
We may employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the intellectual property and other proprietary information, know-how or trade secrets of others in their work for us, we may be subject to claims that we or these employees have used or disclosed such intellectual property or other proprietary information. Litigation may be necessary to defend against these claims.
In addition, while we typically require our employees, consultants and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. To the extent that we fail to obtain such assignments, such assignments do not contain a self-executing assignment of intellectual property rights or such assignments are breached, we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
| 23 |
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance and annuity fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of a patent or patent application include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patent rights and patent applications covering our current or future product candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business, financial condition, results of operations and growth prospects.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations, and growth prospects. In addition, opposition or cancellation proceedings may be filed against our trademark applications and registrations, and our trademarks may not survive such proceedings. In certain countries outside of the United States, trademark registration is required to enforce trademark rights. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would.
If we are unable to protect the confidentiality of our trade secrets, the value of our technology could be materially adversely affected and our business would be harmed.
In addition to seeking patents for important aspects of ANEB-001, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, in seeking to develop and maintain a competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, consultants, independent contractors, advisors, corporate collaborators, outside scientific collaborators, contract manufacturers, suppliers and other third parties. Monitoring unauthorized uses and disclosures is difficult, and we do not know whether the steps we have taken to protect our proprietary technologies will be effective.
| 24 |
While we seek to protect these trade secrets and other proprietary technology, we cannot guarantee that our trade secrets and other proprietary and confidential information will not be disclosed or that competitors will not otherwise gain access to our trade secrets. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts both within and outside the United States may be less willing or unwilling to protect trade secrets. Further, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent such third party, or those to whom they communicate such technology or information, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our business and competitive position could be harmed.
Trade secrets and know-how can be difficult to protect as trade secrets and know-how will over time be disseminated within the industry through independent development, the publication of journal articles, and the movement of personnel skilled in the art from company to company or academic to industry scientific positions. If we fail to prevent material disclosure of the know-how, trade secrets and other intellectual property related to our technologies to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition. Even if we are able to adequately protect our trade secrets and proprietary information, our trade secrets could otherwise become known or could be independently discovered by our competitors. For example, competitors could purchase our product and attempt to replicate some or all of the competitive advantages we derive from our development efforts, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, in the absence of patent protection, we would have no right to prevent them, or those to whom they communicate, from using that technology or information to compete with us.
We may not be able to prevent misappropriation of our intellectual property, trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Intellectual property rights do not necessarily address all potential threats to our business.
Once granted, patents may remain open to opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against such grant. In the course of such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims thus attacked, or may lose the allowed or granted claims altogether. In addition, the degree of future protection afforded by our intellectual property rights is uncertain because even granted intellectual property rights have limitations, and may not adequately protect our business.
The expiration or loss of patent protection may adversely affect our future revenues and operating earnings.
Patent protection is important in the development and eventual commercialization of our product candidate. Patents covering our product candidate normally provide market exclusivity, which is important in order for our product candidate to become profitable. Even if we are successful in obtaining a patent, patents have a limited lifespan. In the United States, the natural expiration of a utility patent is generally 20 years after it is filed. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Without patent protection, we may be open to competition from generic versions of such compositions, methods and devices. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar to ours.
Risks Related to Product Development, Regulatory Approval, Manufacturing and Commercialization
Delays in the completion of, or the termination of, a clinical trial for ANEB-001, our lead drug candidate, could adversely affect our business.
Clinical trials are very expensive, time-consuming, unpredictable and difficult to design and implement. The results of clinical trials may be unfavorable, they may continue for several years, and they may take significantly longer to complete and involve significantly more costs than expected. Delays in the commencement or completion of clinical testing could significantly affect product development costs and plans with respect to our drug candidate. The commencement and completion of clinical trials can be delayed and experience difficulties for a number of reasons, including delays and difficulties caused by circumstances over which we may have no control. For instance, approvals of the scope, design or trial site may not be obtained from the FDA and other required bodies in a timely manner or at all, agreements with acceptable terms may not be reached in a timely manner or at all with contract research organizations, to conduct the trials, a sufficient number of subjects may not be recruited and enrolled in the trials, and third-party manufacturers of the materials for use in the trials may encounter delays and problems in the manufacturing process, including failure to produce materials in sufficient quantities or of an acceptable quality to complete the trials. Clinical trial delays could shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business and results of operation s.
| 25 |
If we are not able to obtain any required regulatory approvals for ANEB-001, we will not be able to commercialize our lead drug candidate and our ability to generate revenue will be limited.
Our drug candidate is a treatment in development for cannabinoid overdose. We must successfully complete clinical trials for our drug candidate before we can apply for marketing approval. Even if we complete our clinical trials, it does not assure marketing approval. Our clinical trials may be unsuccessful, which would materially harm our business. Even if our initial clinical trials are successful, we are required to conduct additional clinical trials to establish our drug candidate’s safety and efficacy, before a New Drug Application (“NDA”) or Biologics License Application (“BLA”), or their foreign equivalents can be filed with the FDA or comparable foreign regulatory authorities for marketing approval of our drug candidate.
Success in early phases of preclinical and clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials can occur at any stage of testing. We may experience unforeseen events during, or as a result of, the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our drug candidate. The research, testing, manufacturing, labeling, packaging, storage, approval, sale, marketing, advertising and promotion, pricing, export, import and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. We are not permitted to market our drug in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. In the United States, the FDA generally requires the completion of clinical trials of each drug to establish its safety and efficacy and extensive pharmaceutical development to ensure its quality before an NDA is approved. Regulatory authorities in other jurisdictions impose similar requirements. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA and even fewer are eventually approved for commercialization. If our development efforts for our drug candidate, including regulatory approval, are not successful for its planned indications, or if adequate demand for our drug candidate is not generated, our business will be materially adversely affected.
Our success depends on the receipt of regulatory approval and the issuance of such regulatory approvals is uncertain and subject to a number of risks, including the following:
| ● | the results of toxicology studies may not support the filing of an Investigational New Drug Application (“IND”) for our drug candidate or the FDA may require additional toxicology studies; | |
| ● | the FDA or comparable foreign regulatory authorities or Institutional Review Boards (“IRB”) may disagree with the design or implementation of our clinical trials; | |
| ● | it may be difficult to run clinical trials involving the administration of THC to subjects because THC is a controlled substance and is illegal in certain jurisdictions; | |
| ● | we may not be able to provide acceptable evidence of our drug candidate’s safety and efficacy; | |
| ● | the results of our clinical trials may not be satisfactory or may not meet the level of statistical or clinical significance required by the FDA or other regulatory agencies for marketing approval; | |
| ● | the dosing of our drug candidate in a particular clinical trial may not be at an optimal level; | |
| ● | patients in our clinical trials may suffer adverse effects for reasons that may or may not be related to our drug candidate; |
| 26 |
| ● | the data collected from clinical trials may not be sufficient to support the submission of an NDA, BLA or other submission or to obtain regulatory approval in the United States or elsewhere; | |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and | |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
Failure to obtain regulatory approval for our drug candidate for the foregoing, or any other reasons, will prevent us from commercializing our drug candidate, and our ability to generate revenue will be materially impaired. We cannot guarantee that regulators will agree with our assessment of the results of the clinical trials we intend to conduct in the future or that such trials will be successful. The FDA and other regulators have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional clinical trials, or preclinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent regulatory approval of our drug candidate.
We have not submitted an NDA or received regulatory approval to market our drug candidate in any jurisdiction. We have only limited experience in filing the applications necessary to gain regulatory approvals and expect to rely on consultants and third party contract research organizations, with expertise in this area to assist us in this process. Securing regulatory approvals to market a product requires the submission of preclinical, clinical, and/or pharmacokinetic data, information about product manufacturing processes and inspection of facilities and supporting information to the appropriate regulatory authorities for each therapeutic indication to establish a drug candidate’s safety and efficacy for each indication. Our drug candidate may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining regulatory approval or prevent or limit commercial use with respect to one or all intended indications.
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon, among other things, the type, complexity and novelty of the drug candidate involved, the jurisdiction in which regulatory approval is sought and the substantial discretion of the regulatory authorities. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for a submitted product application may cause delays in the approval or rejection of an application.
Even if we receive regulatory approval for ANEB-001, our lead drug candidate, we may not be able to successfully commercialize the product and the revenue that we generate from its sales, if any, may be limited.
If approved for marketing, the commercial success of ANEB-001 will depend upon the product’s acceptance by the medical community, including physicians, patients and healthcare payors. The degree of market acceptance for our drug candidate will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; | |
| ● | relative convenience, dosing burden and ease of administration; | |
| ● | the prevalence and severity of any adverse effects; | |
| ● | the willingness of physicians to prescribe our drug candidate, and the target patient population to try new therapies; | |
| ● | efficacy of our drug candidate compared to competing products; | |
| ● | the introduction of any new products that may in the future become available targeting indications for which our drug candidate may be approved; |
| 27 |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our drug candidate may show utility; | |
| ● | pricing and cost-effectiveness; | |
| ● | the inclusion or omission of our drug candidate in applicable therapeutic and vaccine guidelines; | |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; | |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; | |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government healthcare programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and | |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If our drug candidate is approved, but does not achieve an adequate level of acceptance by physicians, healthcare payors and patients, we may not generate sufficient revenue and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our drug candidates may require significant resources and may never be successful.
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our drug candidate successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our drug candidate not commercially viable. For example, regulatory authorities may approve our drug candidate for fewer or more limited indications than we request, may not approve the price we intend to charge for our drug candidate, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve our drug candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that indication. Further, the FDA or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a Risk Evaluation and Mitigation Strategy (“REMS”) to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS; the FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA may also require a REMS for an approved product when new safety information emerges. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of our drug candidate. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our drug candidate.
Even if we obtain marketing approval for ANEB-001, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, ANEB-001 could be subject to labeling and other restrictions and withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with ANEB-001.
Even if we obtain regulatory approval for ANEB-001 for an indication, the FDA or foreign equivalent may still impose significant restrictions on their indicated uses or marketing or the conditions of approval, or impose ongoing requirements for potentially costly and time-consuming post-approval studies and post-market surveillance to monitor safety and efficacy. Our drug candidate will also be subject to ongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping and reporting of adverse events and other post-market information. These requirements include registration with the FDA, as well as continued compliance with current Good Clinical Practices (“GCP”) regulations, for any clinical trials that we conduct post-approval. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current Good Manufacturing Practice (“CGMP”) requirements relating to quality control, quality assurance and corresponding maintenance of records and documents.
| 28 |
The FDA has the authority to require a REMS as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria or requiring patient testing, monitoring and/or enrollment in a registry.
With respect to sales and marketing activities by us or any future partner, advertising and promotional materials must comply with FDA rules in addition to other applicable federal, state and local laws in the United States and similar legal requirements in other countries. In the United States, the distribution of product samples to physicians must comply with the requirements of the U.S. Prescription Drug Marketing Act. Application holders must obtain FDA approval for product and manufacturing changes, depending on the nature of the change. We may also be subject, directly or indirectly through our customers and partners, to various fraud and abuse laws, including, without limitation, the U.S. Anti-Kickback Statute, U.S. False Claims Act, and similar state laws, which impact, among other things, our proposed sales, marketing, and scientific/educational grant programs. If we participate in the U.S. Medicaid Drug Rebate Program, the Federal Supply Schedule of the U.S. Department of Veterans Affairs, or other government drug programs, we will be subject to complex laws and regulations regarding reporting and payment obligations. All of these activities are also potentially subject to U.S. federal and state consumer protection and unfair competition laws. Similar requirements exist in many of these areas in other countries.
If we or a regulatory agency discovers previously unknown problems with our product, such as adverse events of unanticipated severity or frequency, problems with the facility where the product is manufactured, or we or our manufacturers fail to comply with applicable regulatory requirements, we may be subject to the following administrative or judicial sanctions:
| ● | restrictions on the manufacturing or marketing of the product (including complete withdrawal or recall of the product); | |
| ● | warning letters or holds on post-approval clinical trials; | |
| ● | FDA’s refusal to approve pending NDA’s or supplements to approved NDA’s; | |
| ● | suspension or revocation of product license approvals; | |
| ● | product seizures or detentions; | |
| ● | FDA’s refusal to allow imports or exports of products; or | |
| ● | civil penalties, criminal penalties or injunctions. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our drug candidate and generate revenue. Adverse regulatory action, whether pre- or post-approval, can also potentially lead to product liability claims and increase our product liability exposure.
Obtaining and maintaining regulatory approval of ANEB-001 in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of ANEB-001 in other jurisdictions.
Obtaining and maintaining regulatory approval of ANEB-001 in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a drug candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the drug candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials, as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a drug candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
| 29 |
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/ or to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our drug candidate will be harmed.
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize ANEB-001 and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for our drug candidate, restrict or regulate post-approval activities and affect our ability to profitably sell ANEB-001. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our drug candidate, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, the Medicare Modernization Act (“MMA”) changed the way Medicare covers and pays for pharmaceutical products. The MMA expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, the MMA authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for our drug candidate and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
The Affordable Care Act (“ACA”) was intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The ACA revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the law imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
The ACA remains subject to legislative efforts to repeal, modify or delay the implementation of the law. Efforts to date have generally been unsuccessful. If the ACA is repealed or modified, or if implementation of certain aspects of the Health Care Reform Law are delayed, such repeal, modification or delay may materially adversely impact our business, strategies, prospects, operating results or financial condition. We are unable to predict the full impact of any repeal or modification in the implementation of the ACA on us at this time.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. We expect that additional federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, and in turn could significantly reduce the projected value of certain development projects and reduce or eliminate our profitability.
| 30 |
ANEB-001, our lead drug candidate, may face competition sooner than expected.
Our success will depend in part on our ability to obtain and maintain patent protection for important aspects of ANEB-001 and technologies and to prevent third parties from infringing upon our proprietary rights. We must also operate without infringing upon patents and proprietary rights of others, including by obtaining appropriate licenses to patents or other proprietary rights held by third parties, if necessary. However, the applications we have filed or may file in the future may never yield patents that protect our inventions and intellectual property assets. Failure to obtain patents that sufficiently cover our formulations and technologies would limit our protection against compounding pharmacies, outsourcing facilities, generic drug manufacturers, pharmaceutical companies and other parties who may seek to copy our products, produce products substantially similar to ours or use technologies substantially similar to those we own.
If we market ANEB-001, our lead drug candidate, in a manner that violates healthcare fraud and abuse laws, we may be subject to civil or criminal penalties.
The FDA enforces laws and regulations which require that the promotion of pharmaceutical products be consistent with the approved prescribing information. While physicians may prescribe an approved product for a so-called “off label” use, it is unlawful for a pharmaceutical company to promote its products in a manner that is inconsistent with its approved label and any company which engages in such conduct can subject that company to significant liability. Similarly, industry codes in the EU and other foreign jurisdictions prohibit companies from engaging in off-label promotion and regulatory agencies in various countries enforce violations of the code with civil penalties. While we intend to ensure that our promotional materials are consistent with our label, regulatory agencies may disagree with our assessment and may issue untitled letters, warning letters or may institute other civil or criminal enforcement proceedings. In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include the U.S. Anti-Kickback Statute, U.S. False Claims Act and similar state laws. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
We will be completely dependent on third parties to manufacture ANEB-001, and our commercialization of ANEB-001 could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities, fail to provide us with sufficient quantities of ANEB-001 or fail to do so at acceptable quality levels or prices.
We do not currently have, nor do we plan to acquire, the capability or infrastructure to manufacture the API in ANEB-001 for use in our clinical trials or for commercial product, if any. In addition, we do not have the capability to encapsulate our drug candidate as a finished drug product for commercial distribution. As a result, we will be obligated to rely on contract manufacturers, if and when our drug candidate is approved for commercialization. We have not entered into an agreement with any contract manufacturers for commercial supply and may not be able to engage a contract manufacturer for commercial supply of our drug candidate on favorable terms to us, or at all.
The facilities used by our contract manufacturers to manufacture our drug candidate must be approved by the FDA or comparable foreign regulatory authorities pursuant to inspections that will be conducted after we submit an NDA or BLA to the FDA or their equivalents to other relevant regulatory authorities. We will not control the manufacturing process of, and will be completely dependent on, our contract manufacturing partners for compliance with CGMP regulations for manufacture of both active drug substances and finished drug products. These CGMP regulations cover all aspects of the manufacturing, testing, quality control and record keeping relating to our drug candidates. If our contract manufacturers do not successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our drug candidate or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our drug candidate, if approved.
Our contract manufacturers will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with CGMP regulations and similar regulatory requirements. We will not have control over our contract manufacturers’ compliance with these regulations and standards. Failure by any of our contract manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to grant approval to market our drug candidate, delays, suspensions or withdrawals of approvals, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business. In addition, we will not have control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. Failure by our contract manufacturers to comply with or maintain any of these standards could adversely affect our ability to develop, obtain regulatory approval for or market any of our drug candidate.
| 31 |
If, for any reason, these third parties are unable or unwilling to perform, we may not be able to terminate our agreements with them, and we may not be able to locate alternative manufacturers or formulators or enter into favorable agreements with them and we cannot be certain that any such third parties will have the manufacturing capacity to meet future requirements. If these manufacturers or any alternate manufacturer of finished drug product experiences any significant difficulties in its respective manufacturing processes for our API or finished products or should cease doing business with us, we could experience significant interruptions in the supply of our drug candidate or may not be able to create a supply of our drug candidate at all. Were we to encounter manufacturing issues, our ability to produce a sufficient supply of our drug candidate might be negatively affected. Our inability to coordinate the efforts of our third-party manufacturing partners, or the lack of capacity available at our third party manufacturing partners, could impair our ability to supply our drug candidate at required levels. Because of the significant regulatory requirements that we would need to satisfy in order to qualify a new bulk or finished product manufacturer, if we face these or other difficulties with our current manufacturing partners, we could experience significant interruptions in the supply of our drug candidate if we decided to transfer the manufacturing of our drug candidate to one or more alternative manufacturers in an effort to deal with the difficulties.
Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations and result in lost sales. Additionally, we rely on third parties to supply the raw materials needed to manufacture our potential product. Any reliance on suppliers may involve several risks, including a potential inability to obtain critical materials and reduced control over production costs, delivery schedules, reliability and quality. Any unanticipated disruption to a future contract manufacturer caused by problems at suppliers could delay shipment of our drug candidate, increase our cost of goods sold and result in lost sales.
We cannot guarantee that our future manufacturing and supply partners will be able to reduce the costs of commercial scale manufacturing of our drug candidate over time. If the commercial-scale manufacturing costs of our drug candidate are higher than expected, these costs may significantly impact our operating results. In order to reduce costs, we may need to develop and implement process improvements. However, in order to do so, we will need, from time to time, to notify or make submissions with regulatory authorities, and the improvements may be subject to approval by such regulatory authorities.
We cannot be sure that we will receive these necessary approvals or that these approvals will be granted in a timely fashion. We also cannot guarantee that we will be able to enhance and optimize output in our commercial manufacturing process. If we cannot enhance and optimize output, we may not be able to reduce our costs over time.
Any termination or suspension of, or delays in the commencement or completion of, any necessary studies of ANEB-001, our lead drug candidate, for any indications could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects.
The commencement and completion of clinical studies can be delayed for a number of reasons, including delays related to:
| ● | the FDA or a comparable foreign regulatory authority failing to grant permission to proceed and placing the clinical study on hold; | |
| ● | subjects for clinical testing failing to enroll or remain in our trials at the rate we expect; | |
| ● | a facility manufacturing our drug candidate being ordered by the FDA or other government or regulatory authorities to temporarily or permanently shut down due to violations of CGMP requirements or other applicable requirements, or contamination of our drug candidate in the manufacturing process; | |
| ● | any changes to our manufacturing process that may be necessary or desired; | |
| ● | subjects choosing an alternative treatment for the indications for which we are developing our drug candidate, or participating in competing clinical studies; | |
| ● | subjects experiencing severe or unexpected drug-related adverse effects; |
| 32 |
| ● | reports from clinical testing on similar technologies and products raising safety and/or efficacy concerns; | |
| ● | third-party clinical investigators losing their license or permits necessary to perform our clinical trials, not performing our clinical trials on our anticipated schedule or employing methods consistent with the clinical trial protocol, CGMP requirements, or other third parties not performing data collection and analysis in a timely or accurate manner; | |
| ● | inspections of clinical study sites by the FDA, comparable foreign regulatory authorities, or IRB’s finding regulatory violations that require us to undertake corrective action, result in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study, or that prohibit us from using some or all of the data in support of our marketing applications with the FDA; | |
| ● | third-party contractors becoming debarred or suspended or otherwise penalized by the FDA or other government or regulatory authorities for violations of regulatory requirements, in which case we may need to find a substitute contractor, and we may not be able to use some or any of the data produced by such contractors in support of our marketing applications with the FDA; | |
| ● | one or more IRB’s refusing to approve, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; reaching agreement on acceptable terms with prospective contract research organizations and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different contract research organizations and trial sites; | |
| ● | deviations of the clinical sites from trial protocols or dropping out of a trial; | |
| ● | adding new clinical trial sites; | |
| ● | the inability of the contract research organization to execute any clinical trials for any reason; and | |
| ● | government or regulatory delays or “clinical holds” requiring suspension or termination of a trial. |
Product development costs for our drug candidate will increase if we have delays in testing or approval or if we need to perform more or larger clinical studies than planned. Additionally, changes in regulatory requirements and policies may occur and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to the FDA, comparable foreign regulatory authorities, and IRB’s for reexamination, which may impact the costs, timing or successful completion of that study. If we experience delays in completion of, or if we, the FDA or other regulatory authorities, the IRB, or other reviewing entities, or any of our clinical study sites suspend or terminate any of our clinical studies of our drug candidate, its commercial prospects may be materially harmed and our ability to generate product revenues will be delayed. Any delays in completing our clinical trials will increase our costs, slow down our development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical studies may also ultimately lead to the denial of regulatory approval of our drug candidate. In addition, if one or more clinical studies are delayed, our competitors may be able to bring products to market before we do, and the commercial viability of our drug candidate could be significantly reduced.
Clinical drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Clinical testing of our drug candidate is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical testing and early clinical trials may not be predictive of the results of later-stage clinical trials. We cannot assure you that the FDA or comparable foreign regulatory authorities will view the results as we do or that any future trials of our drug candidate will achieve positive results. Drugs in later stages of clinical trials may fail to show the desired safety and efficacy traits despite having progressed through preclinical testing and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles, notwithstanding promising results in earlier trials. Any future clinical trial results for our drug candidate may not be successful.
| 33 |
In addition, a number of factors could contribute to a lack of favorable safety and efficacy results for our drug candidate. For example, such trials could result in increased variability due to varying site characteristics, such as local standards of care and differences in evaluation period, and due to varying patient characteristics including demographic factors and health status.
We may be exposed to product liability risks, and clinical and preclinical liability risks, which could place a substantial financial burden upon us should we be sued.
Our business exposes us to potential product liability and other liability risks that are inherent in the testing, manufacturing and marketing of pharmaceutical formulations and products. We cannot be sure that claims will not be asserted against us. We cannot give assurances that we will be able to continue to obtain or maintain adequate product liability insurance on acceptable terms, if at all, or that such insurance will provide adequate coverage against potential liabilities. A successful liability claim or series of claims brought against us, and any claims or losses in excess of any product liability insurance coverage that we may obtain, could have a material adverse effect on our business, financial condition and results of operations.
ANEB-001, our lead product candidate, may have undesirable side effects which may delay or prevent marketing approval, or, if approval is received, require it to be taken off the market, require it to include safety warnings or otherwise limit sales of the product.
Unforeseen side effects from ANEB-001 could arise either during clinical development or, if approved, after the product has been marketed. This could cause regulatory approvals for, or market acceptance of, the product to be harder and more costly to obtain.
To date, no serious adverse events have been attributed to ANEB-001. The results of our planned or any future clinical trials may show that our product candidate causes undesirable or unacceptable side effects, which could interrupt, delay or halt clinical trials, and result in delay of, or failure to obtain, marketing approval from the FDA and other regulatory authorities, or result in marketing approval from the FDA and other regulatory authorities with restrictive label warnings. If our product candidate receives marketing approval and we or others later identify undesirable or unacceptable side effects caused by the use of our product:
| ● | regulatory authorities may withdraw their approval of the product, which would force us to remove the product from the market; | |
| ● | regulatory authorities may require the addition of labeling statements, specific warnings, a contraindication, or field alerts to physicians, pharmacies and others; | |
| ● | we may be required to change instructions regarding the way the product is administered, conduct additional clinical trials or change the labeling of the product; | |
| ● | we may be subject to limitations on how we may promote the product; | |
| ● | sales of the product may decrease significantly; | |
| ● | we may be subject to litigation or product liability claims; and | |
| ● | our reputation may suffer. |
Any of these events could prevent us or our potential future collaborators from achieving or maintaining market acceptance of the product or could substantially increase commercialization costs and expenses, which in turn could delay or prevent us from generating significant revenues from the sale of our product.
| 34 |
We currently have no marketing and sales organization and we have no direct experience marketing pharmaceutical products. If we are unable to establish our own marketing and sales capabilities, or enter into agreements with third parties to market and sell our products after approval, we may not be able to generate product revenues.
We do not have a sales organization for the marketing, sales and distribution of any pharmaceutical products. In order to commercialize ANEB-001, we must develop these capabilities on our own or make arrangements with third parties for the marketing, sales and distribution of the product. The establishment and development of a direct sales force will be expensive and time-consuming and could delay our product launch, and we cannot be certain that we would be able to successfully develop this capability. As a result, we may seek one or more partners to handle some or all of the sales, marketing and distribution of our product. There also may be certain markets within the United States and elsewhere for our product for which we may seek a co-promotion arrangement. However, we may not be able to enter into arrangements with third parties to sell our product on favorable terms, or at all. In the event, we are unable to develop our own marketing and sales force or collaborate with a third party marketing and sales organization, we will not be able to commercialize our product or any other product candidates that we develop, which will negatively impact our ability to generate product revenues. Furthermore, whether we commercialize our product on our own or rely on a third party, our ability to generate revenue would be dependent on the effectiveness of the sales force. In addition, to the extent we rely on third parties to commercialize our approved product, we would likely receive less revenues than if we commercialized the product ourselves.
New drugs, which may be developed by others, could impair our ability to maintain and grow our business and remain competitive.
The pharmaceutical industry is subject to rapid and substantial technological change. Developments by others may render our technologies and product non-competitive or obsolete. We also may be unable to keep pace with technological developments and other market factors. Technological competition from medical device, pharmaceutical and biotechnology companies, universities, governmental entities and others diversifying into the field is intense and is expected to increase. Many of these entities have significantly greater research and development capabilities and budgets than we do, as well as substantially more marketing, manufacturing, financial and managerial resources. These entities represent significant competition for us.
Our reliance on collaborations with third parties to develop and commercialize ANEB-001 is subject to inherent risks and may result in delays in product development and lost or reduced revenues, restricting our ability to commercialize ANEB-001 and adversely affecting our profitability.
Our ability to develop, obtain regulatory approval of, manufacture and commercialize ANEB-001 depends upon our ability to maintain existing, and enter into and maintain new, contractual and collaborative arrangements with others. We also engage, and intend in the future to continue to engage, contract manufacturers and clinical trial investigators.
In addition, although not a primary component of our current strategy, the identification of new compounds or product candidates for development may require us to enter into license or other collaborative agreements with others, including other pharmaceutical companies and research institutions. Such collaborative agreements for the acquisition of new compounds or product candidates would typically require us to pay license fees, make milestone payments and/or pay royalties. Furthermore, these agreements may result in our revenues being lower than if we developed such product candidates and in our loss of control over the development of such product candidates.
Contractors or collaborators may have the right to terminate their agreements with us or reduce their payments to us under those agreements on limited or no notice and for no reason or reasons outside of our control. For example, we may be unable to maintain our relationship with Vernalis on a commercially reasonable basis, if at all. If we are unable to retain Vernalis as a licensor on commercially acceptable terms, we may not be able to commercialize ANEB-001 and we may experience delays in or suspension of the marketing of our product. The same could apply to other product candidates we may develop or acquire in the future. Our dependence upon third parties to assist with the development and commercialization of our product candidate may adversely affect our ability to generate profits or acceptable profit margins and our ability to develop and deliver such product on a timely and competitive basis.
If our current or future licensees exercise termination rights they may have, or if these license agreements terminate because of delays in obtaining regulatory approvals, or for other reasons, and we are not able to establish replacement or additional research and development collaborations or licensing arrangements, we may not be able to develop and/or commercialize our product candidate. Moreover, any future collaborations or license arrangements we may enter into may not be on terms favorable to us.
| 35 |
A further risk we face with the collaborations is that business combinations and changes in the collaborator or their business strategy may adversely affect their willingness or ability to complete their obligations to us. Our current or any future collaborations or license arrangements ultimately may not be successful. Our agreements with collaborators typically allow them discretion in electing whether to pursue various development, regulatory, commercialization and other activities. If any collaborator were to breach its agreement with us or otherwise fail to conduct collaborative activities in a timely or successful manner, the preclinical or clinical development or commercialization of the affected product candidate or research program would be delayed or terminated.
Other risks associated with our collaborative and contractual arrangements with others include the following:
| ● | we may not have day-to-day control over the activities of our contractors or collaborators; | |
| ● | our collaborators may fail to maintain, defend or enforce patents they own on compounds or technologies that are incorporated into the products we develop with them; | |
| ● | third parties may not fulfill their regulatory or other obligations; and | |
| ● | we may not realize the contemplated or expected benefits from collaborative or other arrangements; and disagreements may arise regarding a breach of the arrangement, the interpretation of the agreement, ownership of proprietary rights, clinical results or regulatory approvals. |
These factors could lead to delays in the development and/or commercialization of our current or future product candidates, or could result in us not being able to commercialize our products. Further, disagreements with our contractors or collaborators could require or result in litigation or arbitration, which would be time-consuming and expensive. Our ultimate success may depend upon the success and performance on the part of these third parties. If we fail to maintain these relationships or establish new relationships as required, development and/or commercialization of our product candidate will be delayed or may never be realized.
Risks Related to Government Regulation of our Industry
Legislative or regulatory reform of the healthcare system may affect our ability to sell our products profitably.
In both the U.S. and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could impact our ability to sell future products and profitability. On March 23, 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively, “PPACA”), which includes a number of healthcare reform provisions and requires most U.S. citizens to have health insurance. The law, among other things, imposes a significant annual fee on companies that manufacture or import branded prescription drug products, addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations, and establishes a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D. Substantial new provisions affecting compliance also have been added, which may require modification of business practices with healthcare practitioners.
In the coming years, additional changes could be made to governmental healthcare programs that could significantly impact the development and success of our future product candidates, and we could be adversely affected by current and future healthcare reforms.
| 36 |
We are subject to regulation from the U.S. Government and such other governments and regulatory bodies where ANEB-001 or future product candidates may be sold in the future.
Both before and after regulatory approval to market a particular product candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, distribution and record keeping related to the product are subject to extensive, ongoing regulatory requirements, including, without limitation, submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with CGMP requirements and GCP requirements for any clinical trials we conduct post-approval. As a result, we are subject to a number of governmental and other regulatory risks, which include:
| ● | clinical development is a long, expensive and uncertain process; delay and failure can occur at any stage of our clinical trials; | |
| ● | our clinical trials are dependent on patient enrollment and regulatory approvals; we do not know whether our planned trials will begin on time, or at all, or will be completed on schedule, or at all; | |
| ● | the FDA or other regulatory authorities may not approve a clinical trial protocol or may place a clinical trial on hold; | |
| ● | we rely on third parties, such as consultants, contract research organizations, medical institutions and clinical investigators, to conduct clinical trials for our drug candidates and if we or any of our third-party contractors fail to comply with applicable regulatory requirements, such as CGMP requirements, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the European Medicines Agency or comparable foreign regulatory authorities may require us to perform additional clinical trials; | |
| ● | if the clinical development process is completed successfully, our ability to derive revenues from the sale of our current or future product candidates will depend on us first obtaining FDA or other comparable foreign regulatory approvals, each of which are subject to unique risks and uncertainties; | |
| ● | there is no assurance that we will receive FDA or corollary foreign approval for any of our product candidates for any indication; we are subject to government regulation for the commercialization of our product candidates; | |
| ● | we have not received regulatory approval in the United States for the commercial sale of our product candidate; | |
| ● | even if our product candidate does obtain approval, regulatory authorities may approve such product candidate for fewer or more limited indications than our requests, may not approve the price we intend to charge for our products, may grant approval contingent on the performance of costly post-marketing clinical trials or may approve with a label that does not include the labeling claims necessary or desirable for the successful commercialization of the product candidate; | |
| ● | undesirable side effects caused by our current or future product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign authorities; | |
| ● | later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with the regulatory requirements of FDA and other applicable United States and foreign regulatory authorities could subject us to administrative or judicially imposed sanctions; | |
| ● | the FDA’s policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our drug candidate, and if we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained; and | |
| ● | we may be liable for contamination or other harm caused by hazardous materials used in the operations of our business. |
| 37 |
In addition, our operations are also subject to various federal and state fraud and abuse, physician payment transparency and privacy and security laws, including, without limitation:
| ● | The federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or providing remuneration to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare or Medicaid programs. This statute has been applied to pharmaceutical manufacturer marketing practices, educational programs, pricing policies and relationships with healthcare providers. A person or entity does not need to have actual knowledge of this statute or specific intent to violate it to have committed a violation; | |
| ● | Federal civil and criminal false claims laws and civil monetary penalty laws, including civil whistleblower or qui tam actions that prohibit, among other things, knowingly presenting, or causing to be present, claims for payment or approval to the federal government that are false or fraudulent, knowingly making a false statement material to an obligation to pay or transmit money or property to the federal government or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay or transmit money or property to the federal government. The government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes; | |
| ● | The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) and its implementing regulations, which created federal criminal laws that prohibit, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; | |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information; | |
| ● | Federal “sunshine” requirements imposed by the PPACA on drug manufacturers regarding any “transfer of value” made or distributed to physicians and teaching hospitals, and any ownership and investment interests held by such physicians and their immediate family members. Failure to submit the required information may result in civil monetary penalties of up an aggregate of $150,000 per year (and up to an aggregate of $1 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests not reported in an annual submission, and may result in liability under other federal laws or regulations; and | |
| ● | State and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require drug manufacturers to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA. |
Many of our business practices are subject to scrutiny by regulatory and government enforcement authorities, as well as to lawsuits brought by private citizens under federal and state laws. Failure to comply with applicable law or an adverse decision in lawsuits may result in adverse consequences to us.
The laws governing our conduct in the U.S., and the conduct of collaborators, licensors or licensees on whom the success of our business relies, are enforceable by administrative, civil, and criminal penalties. Violations of laws such as the FDCA, the Social Security Act (including the Anti-Kickback Statute), and the Federal False Claims Act, and any regulations promulgated under the authority of the preceding, may result in a range of enforcement action including jail sentences, fines integrity oversight and reporting obligations and/or exclusion from federal and state healthcare programs, as may be determined by Medicare, Medicaid and the Department of Health and Human Services and other regulatory authorities as well as by the courts in response to actions brought by the Department of Justice. The FDA regulates drugs throughout the development process, from preclinical and clinical trials through approval and post-marketing requirements. Failure to fully comply with FDA law may cause the FDA to issue inspectional observations, untitled or warning letters, bring an enforcement action, suspend or withdraw an approved product from the market, require a recall or institute fines or civil fines, or could result in disgorgement of money, operating restrictions, injunctions or criminal prosecution, any of which (whether applied directly to us or to our collaborators, licensors, or licensees) could harm our reputation and our business. There can be no assurance that our activities, or those of our collaborators, licensors or licensees, will not come under the scrutiny of regulators and other government authorities or that our practices will not be found to violate applicable laws, rules and regulations or prompt lawsuits by private citizen “relators” under federal or state false claims laws.
| 38 |
Clinical trials for ANEB-001 have or may in the future be conducted outside the United States and not under an IND, and where this is the case, the FDA may not accept data from such trials.
Although the FDA may accept data from clinical trials conducted outside the U.S. and not under an IND in support of research or marketing applications for our product candidates, this is subject to certain conditions set out in 21 C.F.R. § 312.120. For example, such foreign clinical trials should be conducted in accordance with GCP, including review and approval by an independent ethics committee and obtaining the informed consent from subjects of the clinical trials. The FDA must also be able to validate the data from the study through an onsite inspection if the agency deems it necessary. The foreign clinical data should also be applicable to the U.S. population and U.S. medical practice. Other factors that may affect the acceptance of foreign clinical data include differences in clinical conditions, study populations or regulatory requirements between the U.S. and the foreign country.
Laws impacting the U.S. healthcare system are subject to a great deal of uncertainty, which may result in adverse consequences to our business.
There have been a number of legislative and regulatory proposals to change the healthcare system, reduce the costs of healthcare and change medical reimbursement policies. Doctors, clinics, hospitals and other users of our product may decline to purchase our product to the extent there is uncertainty regarding coverage from government or commercial payors. Further proposed legislation, regulation and policy changes affecting third-party reimbursement are likely. Among other things, Congress has in the past proposed changes to and the repeal of the PPACA, and lawsuits have been brought challenging aspects of the law at various points. There have been repeated recent attempts by Congress to repeal or replace the PPACA. Some of the provisions of the PPACA have yet to be implemented, and there have been legal and political challenges to certain aspects of the PPACA. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the PPACA. Concurrently, Congress has considered legislation that would repeal and replace all or part of the PPACA. While Congress has previously been successful at passing comprehensive repeal legislation through both Chambers of Congress, it had then been vetoed by former President Obama and full repeal legislation is unlikely in the current political climate. However, Congress has passed two bills affecting the implementation of certain taxes under the PPACA. The Tax Cuts and Jobs Act passed in December of 2017 included a provision that would repeal one of the primary pillars of the law, the PPACA’s individual mandate penalty that essentially assessed a monetary penalty or fine on certain individuals who fail to maintain qualifying health coverage for all or part of a year. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain fees mandated by the PPACA, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans and the annual fee imposed on certain health insurance providers based on market share. Moreover, the Bipartisan Budget Act of 2018 among other things, amends the PPACA, effective January 1, 2019, to increase from 50% to 70% the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”. Congress may consider other legislation to repeal or replace elements of the PPACA on a provision-by-provision basis. In addition, there have been recent congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, control drug costs, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. We are unable to predict what legislation or regulation, if any, relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future at the state or federal level, or what effect such legislation or regulation may have on us. Denial of coverage and reimbursement of our products, or the revocation or changes to coverage and reimbursement policies, could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Ownership of Our Common Stock and this Offering
Our stock price may be volatile and your investment could decline in value.
The market price of our common stock following this offering may fluctuate substantially as a result of many factors, some of which are beyond our control. These fluctuations could cause you to lose all or part of the value of your investment in our common stock. Factors that could cause fluctuations in the market price of our common stock include the following:
| ● | quarterly variations in our results of operations; |
| 39 |
| ● | results of operations that vary from the expectations of securities analysts and investors; | |
| ● | results of operations that vary from those of our competitors; | |
| ● | changes in expectations as to our future financial performance, including financial estimates by securities analysts; | |
| ● | publication of research reports about us or the pharmaceutical industry; | |
| ● | announcements by us or our competitors of significant contracts, acquisitions or capital commitments; | |
| ● | announcements by third parties of significant claims or proceedings against us; | |
| ● | changes affecting the availability of financing in the wholesale and consumer lending markets; | |
| ● | regulatory developments in the pharmaceutical industry; | |
| ● | significant future sales of our common stock, and additions or departures of key personnel; | |
| ● | the realization of any of the other risk factors presented in this prospectus; and | |
| ● | general economic, market and currency factors and conditions unrelated to our performance. |
In addition, the stock market in general has experienced significant price and volume fluctuations that have often been unrelated or disproportionate to operating performance of individual companies. These broad market factors may seriously harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted. A class action suit against us could result in significant liabilities and, regardless of the outcome, could result in substantial costs and the diversion of our management’s attention and resources.
Our common stock has no prior market and our stock price may decline after the offering.
Before this offering, there has been no public market for shares of our common stock. Although we have applied to have our common stock listed on The Nasdaq Capital Market, an active trading market for our common stock may not develop or, if it develops, may not be sustained after this offering. Our company and the underwriters will negotiate to determine the initial public offering price. The initial public offering price may be higher than the market price of our common stock after the offering and you may not be able to sell your shares of our common stock at or above the price you paid in the offering. As a result, you could lose all or part of your investment.
Investors purchasing common stock in this offering will experience immediate dilution.
The initial public offering price of shares of our common stock is higher than the pro forma as adjusted net tangible book value per outstanding share of our common stock. You will incur immediate dilution of $ per share in the pro forma as adjusted net tangible book value of shares of our common stock, based on an assumed initial public offering price of $ per share, the midpoint of the estimated offering price range set forth on the cover page of this prospectus. To the extent outstanding options are ultimately exercised, there will be further dilution of the common stock sold in this offering.
Future sales, or the perception of future sales, of a substantial number of our shares of common stock could depress the trading price of our common stock.
If we or our stockholders sell substantial numbers of our shares of common stock in the public market following this offering or if the market perceives that these sales could occur, the market price of shares of our common stock could decline. These sales may make it more difficult for us to sell equity or equity-linked securities in the future at a time and price that we deem appropriate, or to use equity as consideration for future acquisitions.
| 40 |
Immediately upon completion of this offering, based on the number of shares outstanding as of December 31, 2020, we will have shares of common stock authorized and shares of common stock outstanding. Of these shares, the shares to be sold in this offering (assuming the underwriters do not exercise their option to purchase additional shares in this offering to cover over-allotments, if any) will be freely tradable. We, our executive officers and directors, and certain of our stockholders have entered into agreements with the underwriters not to sell or otherwise dispose of shares of our common stock for a period of 180 days following completion of this offering, with certain exceptions. Immediately upon the expiration of this lock-up period, shares will be freely tradable pursuant to Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”), by non-affiliates and another shares will be eligible for resale pursuant to Rule 144 under the Securities Act, subject to the volume, manner of sale, holding period and other limitations of Rule 144.
In addition, following the completion of this offering, we intend to file a registration statement on Form S-8 registering the issuance of 275,000 shares of common stock subject to stock options and other equity awards issued or reserved for future issuance under our 2020 Stock Incentive Plan. After taking into consideration the 163,750 shares of restricted common stock awarded to Dr. Schneeberger and shares of common stock reserved for issuance upon the exercise of stock options awarded to our non-employee directors in 2021, the Plan has shares remaining available. Shares registered under the registration statement on Form S-8 will be available for sale in the public market subject to vesting arrangements and exercise of options, the lock-up agreements described above and the restrictions of Securities Act Rule 144 in the case of our affiliates.
Changes in accounting principles or guidance, or in their interpretations, could result in unfavorable accounting charges or effects, including changes to our previously filed financial statements, which could cause our stock price to decline.
We prepare our financial statements in accordance with accounting principles generally accepted in the United States of America. These principles are subject to interpretation by the SEC and various bodies formed to interpret and create appropriate accounting principles and guidance. A change in these principles or guidance, or in their interpretations, may have a significant negative effect on our reported results and retroactively affect previously reported results, which, in turn, could cause our stock price to decline.
We will incur significantly increased costs as a result of operating as a public company, and our management will be required to devote substantial time to compliance efforts.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. For example, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the accounting and internal controls provisions of the Foreign Corrupt Practices Act of 1977, as amended, and will be required to comply with the applicable requirements of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), and the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (the “Dodd-Frank Act”), as well as rules and regulations subsequently implemented by the SEC and Nasdaq, including the establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel will need to devote a substantial amount of time and resources to complying with these requirements. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. In particular, we expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act, which will increase when we are no longer an “emerging growth company,” as defined by the JOBS Act. These new obligations will require substantial attention from our management team and could divert their attention away from the day-to-day management of our business. We will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge and maintain an internal audit function. We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors and board committees or as executive officers, and more expensive for us to obtain director and officer liability insurance.
We are an “emerging growth company” and our election to delay adoption of new or revised accounting standards applicable to public companies may result in our financial statements not being comparable to those of some other public companies. As a result of this and other reduced disclosure requirements applicable to emerging growth companies, our securities may be less attractive to investors.
As a company with less than $1.07 billion in annual revenue, we qualify as an “emerging growth company” under the JOBS Act. An emerging growth company may take advantage of specified reduced reporting requirements that are otherwise generally applicable to public companies. In particular, as an emerging growth company we:
| ● | are not required to obtain an attestation and report from our auditors on our management’s assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act; |
| 41 |
| ● | are not required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion and analysis”); | |
| ● | are not required to obtain a non-binding advisory vote from our stockholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute” votes); | |
| ● | are exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; | |
| ● | may present only two years of audited financial statements and only two years of related Management’s Discussion & Analysis of Financial Condition and Results of Operations (“MD&A”); and | |
| ● | are eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. |
We intend to take advantage of all of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under §107 of the JOBS Act.
Certain of these reduced reporting requirements and exemptions were already available to us due to the fact that we also qualify as a “smaller reporting company” under SEC rules. For instance, smaller reporting companies are not required to obtain an auditor attestation and report regarding management’s assessment of internal control over financial reporting, are not required to provide a compensation discussion and analysis, are not required to provide a pay-for-performance graph or CEO pay ratio disclosure, and may present only two years of audited financial statements and related MD&A disclosure.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act, or such earlier time that we no longer meet the definition of an emerging growth company. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1.07 billion in annual revenue, have more than $700 million in market value of our common stock held by non-affiliates, or issue more than $1 billion in principal amount of non-convertible debt over a three-year period. Under current SEC rules, however, we will continue to qualify as a “smaller reporting company” for so long as we have a public float (i.e., the market value of common equity held by non-affiliates) of less than $250 million as of the last business day of our most recently completed second fiscal quarter.
We cannot predict if investors will find our securities less attractive due to our reliance on these exemptions. If investors were to find our securities less attractive as a result of our election, we may have difficulty raising all of the proceeds we seek in this offering.
While we currently qualify as an “emerging growth company” under the JOBS Act, once we lose emerging growth company status, the costs and demands placed upon our management are expected to increase.
Following this offering, we will continue to be an emerging growth company until the earliest to occur of (i) the last day of the fiscal year during which we had total annual gross revenue of at least $1.07 billion (as indexed for inflation), (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of common stock under this registration statement, (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt, or (iv) the date on which we are deemed to be a “large accelerated filer,” as defined under the Exchange Act. Once we lose emerging growth company status, we expect the costs and demands placed upon our management to increase, as we would have to comply with additional disclosure and accounting requirements.
| 42 |
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they change their recommendations regarding our stock adversely, or if our actual results differ significantly from our guidance, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us change their recommendation regarding our stock adversely, or provide more favorable relative recommendations about our competitors, our stock price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
In addition, from time to time, we may release earnings guidance or other forward-looking statements in our earnings releases, earnings conference calls or otherwise regarding our future performance that represent our management’s estimates as of the date of release. Some or all of the assumptions of any future guidance that we furnish may not materialize or may vary significantly from actual future results. Any failure to meet guidance or analysts’ expectations could have a material adverse effect on the trading price or volume of our stock.
Anti-takeover provisions in our charter documents could discourage, delay or prevent a change in control of our company and may affect the trading price of our common stock.
Our corporate documents and Delaware corporate law contain provisions that may enable our board of directors to resist a change in control of our company even if a change in control were to be considered favorable by you and other stockholders. These provisions:
| ● | authorize the issuance of “blank check” preferred stock that could be issued by our board of directors to help defend against a takeover attempt; | |
| ● | provide that vacancies on our board of directors, including vacancies as a result of removal or enlargement of the board of directors, may be filled by directors then in office, even though less than a quorum; | |
| ● | establish that our board of directors is divided into three classes, with each class serving three-year staggered terms; | |
| ● | specify that special meetings of our stockholders can be called only by our board of directors, chief executive officer, or the chairman of our board of directors; | |
| ● | establish an advance notice procedure for stockholder proposals to be brought before an annual meeting, including proposed nominations of persons for election to our board of directors; | |
| ● | include a forum selection clause, which means certain litigation can only be brought in Delaware; and | |
| ● | require supermajority stockholder voting to effect certain amendments to our certificate of incorporation and bylaws. |
In addition, Delaware corporate law prohibits large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or consolidating with us except under certain circumstances. These provisions and other provisions under Delaware corporate law could discourage, delay or prevent a transaction involving a change in control of our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing and cause us to take other corporate actions you desire.
Our certificate of incorporation provides that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for substantially all disputes between us and our stockholders, and federal district courts will be the sole and exclusive forum for Securities Act claims, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by our directors, officers or other employees to us or to our stockholders, (iii) any action asserting a claim against us or any director, officer or other employee arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine, in all cases to the fullest extent permitted by law and subject to the court having personal jurisdiction over the indispensable parties named as defendants; provided that these provisions of our certificate of incorporation will not apply to suits brought to enforce a duty or liability created by the Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction.
Our certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act, unless we consent in writing to the selection of an alternative forum. We note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. The choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our current or former directors, officers, or other employees or stockholders, which may discourage such lawsuits against us and our current or former directors, officers, and other employees or stockholders. Alternatively, if a court were to find the choice of forum provisions contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, financial condition and results of operations.
Concentration of ownership of our common stock by Dr. Joseph F. Lawler and Aron R. English may limit new investors from influencing significant corporate decisions.
Upon completion of this offering, Joseph F. Lawler, M.D., Ph.D., our founder and a director, and Aron R. English, a director of our company, will beneficially own approximately % of our outstanding shares of common stock. These majority stockholders, acting together, would be able to determine the outcome of all matters requiring stockholder approval, including the election and removal of directors and any merger or other significant corporate transactions. The interests of these majority stockholders may not align with our interests or the interests of other stockholders and thereby could control our policies and operations, including the appointment of management, future issuances of our common stock, preferred stock or other securities, the incurrence or modification of debt by us, amendments to our certificate of incorporation and bylaws, and the entering into of extraordinary transactions, such as a merger or sale of all or substantially all of our assets. In addition, these majority stockholders will be able to cause or prevent a change in control of our company and could preclude any unsolicited acquisition of our company. This concentration of ownership could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of our company and ultimately might affect the market price of our common stock.
| 43 |
We may invest or spend the proceeds of this offering in ways with which you may not agree or in ways that may not yield a return.
Our management will have considerable discretion in the application of the net proceeds of this offering, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. The net proceeds may be invested with a view towards long-term benefits for our stockholders and this may not increase our operating results or market value. Until the net proceeds are used, they may be placed in investments that do not produce significant income or that may lose value.
We do not expect to pay any dividends on our common stock for the foreseeable future.
We currently expect to retain all future earnings, if any, for future operation, expansion and debt repayment and have no current plans to pay any cash dividends to holders of our common stock for the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of our board of directors and will depend on, among other things, our operating results, financial condition, cash requirements, contractual restrictions and other factors that our board of directors may deem relevant. In addition, we must comply with the covenants in our credit agreements in order to be able to pay cash dividends, and our ability to pay dividends generally may be further limited by covenants of any existing and future outstanding indebtedness we or our subsidiaries incur. As a result, you may not receive any return on an investment in our common stock unless you sell our common stock for a price greater than that which you paid for it.
| 44 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve substantial risks and uncertainties. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” but are also contained in other sections of this prospectus. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “aim,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing,” “target,” “seek” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
| ● | our limited operating history; | |
| ● | the expectation that we will incur operating losses for the foreseeable future; | |
| ● | our current and future capital requirements to support our development and commercialization efforts for ANEB-001 and our ability to satisfy our capital needs; | |
| ● | our dependence on our lead product candidate, ANEB-001, which is still in an early stage of clinical development; | |
| ● | our reliance on a license from a third party in relation to our rights and development of ANEB-001; | |
| ● | our, or that of our future third-party manufacturers, ability to manufacture GMP batches of our product as required for preclinical and clinical trials and, subsequently, our ability to manufacture commercial quantities of our product; | |
| ● | our ability to attract and retain key executives and medical and scientific personnel; | |
| ● | our ability to complete required clinical trials for ANEB-001 and obtain approval from the FDA or other regulatory agencies in different jurisdictions; | |
| ● | our lack of a sales and marketing organization and our ability to commercialize our product candidates if we obtain regulatory approval; |
| 45 |
| ● | our dependence on third parties to manufacture our product candidates; | |
| ● | our reliance on third-party contract research organizations to conduct our clinical trials; | |
| ● | our ability to maintain and protect the validity of our intellectual property and develop new intellectual property; | |
| ● | interpretations of current laws and the passages of future laws; | |
| ● | acceptance of our business model by investors; | |
| ● | the accuracy of our estimates regarding expenses and capital requirements; and | |
| ● | our ability to adequately support organizational and business growth. |
We caution you that the foregoing list may not contain all of the forward-looking statements made in this prospectus.
These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain.
You should refer to the “Risk Factors” section of this prospectus for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result, of these factors, we cannot assure you that the forward-looking statements in this prospectus will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by U.S. federal securities laws.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
| 46 |
We estimate that the net proceeds from the sale of our common stock in this offering will be approximately $ million (or approximately $ million if the underwriters exercise their option in full to purchase additional shares of our common stock from us), based upon the assumed initial public offering price of $ per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase or decrease in the assumed initial public offering price of $ per share, which is the midpoint of the estimated offering price range set forth on the cover page of this prospectus, would increase or decrease the net proceeds that we receive from this offering by approximately $ , assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds from this offering to make expenditures to fund proprietary research and development of our ANEB-001 product candidate and to support preclinical testing and clinical trials necessary for regulatory filings. The amount and timing of expenditures for these purposes will vary depending upon a number of factors, none of which can be predicted with certainty, such as the progress of research and development projects, participation of strategic partners, changing competitive conditions, technological advances, patent considerations and assessments of the commercial potential of our products.
We believe that we will receive sufficient net proceeds from this offering to complete the P2 proof-of-concept trial, advance regulatory discussions with the FDA and comparable foreign regulatory bodies and prepare for pivotal clinical trials that focus on the safety of our ANEB-001 product candidate. As of the date of this prospectus, we have not determined the amount of net proceeds of this offering to be applied specifically to each of these uses. We will need additional funding to complete the clinical development of, seek regulatory approval for and commercially launch ANEB-001 and other pipeline development products. In particular, we expect that we will need additional capital in approximately 18 months to run the pivotal safety trials for ANEB-001, file a marketing application with the FDA and make certain milestone payments to Vernalis (as described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Contractual Obligations and Commitments”). Additionally, we will require financing if we decide to commercialize ANEB-001 without any future third-party collaborative arrangements.
A portion of the net proceeds from this offering may be used for the acquisition or licensing of complementary technologies, products or businesses. We currently have no commitments or understandings to make any such acquisitions or enter into any new licenses.
The net proceeds from this offering will also be available for working capital and other general corporate purposes, including enhancing our corporate infrastructure and systems to assist in creating a more robust means of tracking data, automating back office functions and improving our financial reporting system. We may allocate funds from other sources to fund some or all of these activities.
The intended use of net proceeds from this offering represents our expectations based upon our present plans and business conditions. We cannot predict with certainty all of the particular uses for the proceeds of this offering or the amounts that we will actually spend on the uses described in this prospectus. Accordingly, our management will have significant flexibility in applying the net proceeds of this offering. The timing and amount of our actual expenditures will be based on many factors, including cash flows from operations, if any, and the anticipated growth of our business. Pending such uses, we intend to invest the net proceeds of this offering in a variety of capital-preservation investments, including short- and intermediate-term, interest-bearing, investment-grade securities.
| 47 |
Our board of directors will determine our future dividend policy based on our results of operations, financial condition, capital requirements and other circumstances. We have not previously declared or paid any cash dividends on our common stock. We anticipate that we will retain earnings to support operations and finance the growth of our business, as described in this prospectus. Accordingly, it is not anticipated that any cash dividends will be paid on our common stock in the foreseeable future.
| 48 |
The following table sets forth our cash and cash equivalents and total capitalization as of December 31, 2020 on:
| ● | an actual basis without any adjustments to reflect subsequent or anticipated events; | |
| ● | a pro forma basis reflecting the receipt by us of the proceeds from the sale and exercise of our milestone warrants for a total of $ before the closing of this offering, and the conversion of all our series A preferred stock into shares of common stock automatically upon the closing of this offering (the “Recapitalization”); and | |
| ● | a pro forma as adjusted basis reflecting the Recapitalization and the receipt by us of the net proceeds from the sale of shares of our common stock in this offering at the assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and excluding the exercise of the underwriters’ over-allotment option, as if each had occurred on December 31, 2020. |
The pro forma and pro forma as-adjusted information below is illustrative only of our cash and cash equivalents and capitalization following the completion of this offering and will change based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with the information set forth in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this prospectus.
| As of December 31, 2020 | ||||||||||||
| Actual | Pro Forma | Pro Forma, as Adjusted | ||||||||||
| (unaudited) | (unaudited) | |||||||||||
| Cash and cash equivalents | $ | 2,480,003 | $ | |||||||||
| Series A preferred stock, $0.0001 par value, 1,490,651 shares authorized; 341,250 shares issued and outstanding; no shares issued and outstanding, pro forma, as adjusted | 2,975,752 | |||||||||||
| Stockholders’ equity (deficit) | ||||||||||||
| Common stock, $0.001 par value, 3,800,000 shares authorized; 2,163,750 shares issued and outstanding; shares issued and outstanding, pro forma, as adjusted | 2,164 | |||||||||||
| Additional paid-in capital | 38,438 | |||||||||||
| Accumulated deficit | (759,620 | ) | ||||||||||
| Total stockholders’ equity (deficit) | (719,018 | ) | ||||||||||
| Total capitalization | $ | 2,256,734 | $ | |||||||||
The number of shares of common stock issued and outstanding actual, pro forma and pro forma as adjusted in the table above excludes the following:
| ● | the sale of our milestone warrants and the conversion of all our series A preferred stock into shares of common stock automatically upon the closing of this offering (see “Prospectus Summary – Private Placement and Recapitalization”); | |
| ● | _____ shares of common stock reserved for issuance upon the exercise of outstanding stock options awarded to our non-employee directors in 2021, and _____ shares of common stock reserved for future issuance under our 2020 Stock Incentive Plan; and | |
| ● | the exercise by the underwriters of their option to purchase up to an additional shares of our common stock from us in this offering to cover over-allotments, if any. |
Each $1.00 increase or decrease in the assumed initial public offering price of $ per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, would increase or decrease the total consideration paid by new investors purchasing shares of our common stock in this offering by $ , assuming that the number of shares of our common stock offered by us, as set forth on the cover page of this prospectus, remains the same.
| 49 |
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share and the net tangible book value per share of our common stock immediately after this offering. Net tangible book value per share is determined by dividing our total tangible assets less total liabilities by the number of outstanding shares of common stock.
As of December 31, 2020, we had a net tangible book value of $2,126,228, or $0.98 per share of common stock. Our net tangible book value per share represents the amount of our total tangible assets reduced by the amount of our total liabilities and divided by the total number of shares of our common stock outstanding as of December 31, 2020.
Investors participating in this offering will incur immediate and substantial dilution. After giving effect to the sale and exercise of our milestone warrants for a total of $2,250,000 before the closing of this offering and the conversion of all our series A preferred stock into shares of common stock automatically upon the closing of this offering, and issuance and sale of shares of our common stock in this offering at the assumed initial public offering price of $ per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our net tangible book value as of December 31, 2020, would have been approximately $ , or $ per share of common stock. This represents an immediate increase in the pro forma net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to investors purchasing shares of our common stock in this offering. The following table illustrates this per share dilution:
| Amount | ||||
| Assumed initial public offering price per share | $ | |||
| Net tangible book value (deficit) per share as of December 31, 2020 | ||||
| Increase in net tangible book value per share attributable to new investors participating in this offering | ||||
| Net tangible book value per share after this offering | ||||
| Dilution per share to new investors participating in this offering | ||||
The dilution information discussed above is illustrative only and will change based on the actual initial public offering price and other terms of this offering determined at pricing. Each $1.00 increase or decrease in the assumed initial public offering price of $ per share, which is the midpoint of the estimated offering price set forth on the cover page of this prospectus, would increase or decrease our net tangible book value by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
If the underwriters exercise their over-allotment option in full to purchase an additional shares of our common stock from us in this offering to cover over-allotments, if any, the pro forma as adjusted net tangible book value per share of our common stock after giving effect to this offering, would be $ per share, the increase in the net tangible book value per share to existing stockholders would be $ per share and the dilution per share to new investors purchasing shares of our common stock in this offering would be $ per share.
The following table illustrates, as of December 31, 2020, the differences between the number of shares of common stock purchased from us, the total consideration paid to us, and the average price per share paid by existing stockholders and by new investors purchasing shares of our common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, and before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Shares Purchased | Total Consideration | Average Price Per | ||||||||||||||||||
| Number | Percent | Amount | Percent | Share | ||||||||||||||||
| Existing stockholders | % | % | ||||||||||||||||||
| New investors | % | % | ||||||||||||||||||
| Total | 100.0 | % | 100.0 | % | ||||||||||||||||
| 50 |
Each $1.00 increase or decrease in the assumed initial public offering price of $ per share, which is the midpoint of the estimated price range set forth on the cover page of this prospectus, would increase or decrease the total consideration paid by new investors purchasing shares of our common stock in this offering by $ , assuming that the number of shares of our common stock offered by us, as set forth on the cover page of this prospectus, remains the same.
The number of shares of our common stock shown above to be outstanding after this offering is based on shares of our common stock outstanding as of December 31, 2020. After taking into consideration the 163,750 shares of restricted common stock awarded to Dr. Schneeberger and shares of common stock reserved for issuance upon the exercise of outstanding stock options awarded to our non-employee directors in 2021, the Plan has shares remaining available.
In addition, if the underwriters exercise their over-allotment option in full to purchase additional shares of our common stock from us in this offering, the number of shares held by new investors purchasing shares of our common stock in this offering would increase to , or % of the total number of shares of our common stock outstanding after this offering.
To the extent that new options are issued under our 2020 Stock Incentive Plan or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
| 51 |
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes included elsewhere in this prospectus. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included elsewhere in this prospectus.
Overview
We are a clinical-stage biotechnology company developing novel solutions for people suffering from cannabinoid overdose and substance addiction. Our lead product candidate, ANEB-001, is intended to reverse the negative effects of cannabinoid overdose within 1 hour of administration. The signs and symptoms of cannabinoid overdose range from profound sedation to anxiety and panic to psychosis with hallucinations. There is no approved medical treatment currently available to specifically alleviate the symptoms of cannabinoid overdose. If approved by the FDA, we believe ANEB-001 has the potential to be the first FDA approved treatment of its kind on the market for reversing the effects of THC, the principal psychoactive constituent of cannabis. Clinical trials completed to date have shown that ANEB-001 is rapidly absorbed, well tolerated and leads to weight loss, an effect that is consistent with central CB1 antagonism. We intend to launch a Phase 2 proof - of - concept trial for ANEB-001 in the fourth quarter of 2021.
Cannabinoid overdoses have become a widespread health issue in the United States, particularly in the increasing number of states that have legalized cannabis for personal and recreational use. The ingestion of large quantities of THC is a major cause of cannabinoid overdose. Excessive ingestion of THC via edible products such as candies and brownies, and overdoses of synthetic cannabinoids (also known as “synthetics,” “K2” or “spice”), are two leading causes of THC-related emergency room visits. Synthetic cannabinoids are analogous to fentanyl for opioids insofar as they are more potent at the cannabinoid receptor than their natural product congener THC. In recent years, hospital emergency rooms across the United States have seen a dramatic increase in patient visits with cannabis-related conditions. Before the legalization of cannabis, an estimated 450,000 patients visited hospital emergency rooms for cannabis-related conditions. In 2014, this number more than doubled to an estimated 1.1 million patients, according to data published in “Trends and Related Factors of Cannabis-Associated Emergency Department Visits in the United States: 2006-2014,” Journal of Addiction Medicine (May/June 2019), which provided a national estimate analyzing data from The Nationwide Emergency Department Sample (NEDS), the largest database of U.S. hospital-owned emergency department visits. Based on our own analysis of the most recent NEDS data, we believe that the number of hospitalizations grew to 1.74 million patients in 2018 and was growing at an approximately 15% compounded annual growth rate between 2012 and 2018. We believe the number of cannabis-related hospitalizations and other health problems associated with cannabinoid overdoses such as depression, anxiety and mental disorders will continue to increase substantially as more states pass laws legalizing cannabis for medical and recreational use. Given the consequences, there is an urgent need for a treatment to rapidly reverse the symptoms of cannabinoid overdose.
In May 2020, we entered into a royalty-bearing license agreement with Vernalis R&D Limited (“License Agreement”) to exploit its license compounds and licensed products to combat symptoms of cannabinoid overdose and substance addiction. We are currently developing our lead product candidate, ANEB-001 to quickly, and effectively, combat symptoms of cannabinoid overdose.
Our objective is to develop and commercialize new treatment options for patients suffering from cannabinoid overdose and addiction. Our lead product candidate is ANEB-001, a potent, small molecule cannabinoid receptor antagonist, to address the unmet medical need for a specific antidote for cannabinoid overdose. ANEB-001 is an orally bioavailable, rapidly absorbed treatment that we anticipate will reverse the symptoms of cannabinoid overdoses, in most cases within 1 hour of administration. Our proprietary position in the treatment of cannabinoid overdose is protected by rights to two patent applications covering various methods of use of the compound and delivery systems. We anticipate starting a Phase 2 proof-of-concept trial for ANEB-001 in the fourth quarter of 2021.
We were formed on April 23, 2020, incorporated in Delaware in April 2020, and commenced operations in May 2020. Our operations to date have consisted of organizing and acquiring the license rights to Vernalis’ licensed products, assembling an executive team, starting preparations for a Phase 2 proof-of-concept trial, including the synthesis of a new active pharmaceutical ingredient, the development and filing of a clinical trial protocol with regulatory agencies in Europe and raising capital. We have funded our operations through a private placement of our series A convertible preferred stock and issuance of two promissory notes to a related party.
We have not generated any revenue from product sales since inception. We expect to continue incurring significant research and development expenses related to ANEB-001. We have incurred operating losses since inception and expect to continue to incur significant operating losses and negative cash flows from operations for the foreseeable future. For the period from April 23, 2020 (inception) to June 30, 2020 and as of June 30, 2020, we r ecorded a net loss of $174,637 and had cash and cash equivalents of $3,024,980 and accumulated deficit of $174,637, respectively. For the six months ended December 31, 2020 and as of December 31, 2020, we recorded a net loss of $584,983 and accumulated deficit of $759,620, respectively.
As of December 31, 2020, our cash and cash equivalents were $2,480,003. In June 2020, we raised $3,200,000 in gross proceeds from the sale of our series A preferred and issuance of the related party notes. We believe that our existing cash and cash equivalents, together with the anticipated net proceeds from this offering, will enable us to fund our commercialization efforts, operating expenses, clinical trials, product development and capital requirements for at least the next 18 months. We will need to raise additional capital in order to complete the clinical development of, seek regulatory approval for and commercially launch ANEB-001 and other pipeline development products. See “– Liquidity and Capital Resources” below.
| 52 |
We expect our expenses and operating losses to increase substantially and will include:
| ● | employee-related expenses, such as salaries, share-based compensation, benefits, travel expenses for personnel that we plan to hire; | |
| ● | manufacturing costs in connection with conducting clinical trials; | |
| ● | expenses related to planned clinical trials; | |
| ● | earlier stage research and development activities; | |
| ● | costs associated with protecting our intellectual property; | |
| ● | costs to acquire or in-license other assets and technologies; and | |
| ● | additional costs associated with being a public company. |
In addition, as we progress forward, we will be obligated to make certain milestone payments to the licensor. See “– Contractual Obligations and Commitments” below. Our net losses may fluctuate significantly quarterly or yearly, depending on the timing of development milestone expenses, clinical trials, research and development expenditures and commercialization expenses.
We will need to raise additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from sales of ANEB-001, if approved, we expect to finance our operations through the sale of equity securities, debt financings or other capital resources, including potential collaborations with third parties or other strategic transactions. Adequate funding may not be available to us on acceptable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, scale back or discontinue the development of ANEB-001 or additional indications on product candidates we may develop in the future.
Financial Operations Overview
Revenue
We have not generated any revenue since inception. If our development efforts for our current lead product candidate, ANEB-001, or other additional product candidates that we may develop in the future, are successful and result in marketing approval, or if we enter into collaboration or license agreements with third parties, we may generate revenue in the future from a combination of product sales or payments from such collaboration or license agreements. We cannot predict if, when, or to what extent we will generate revenue from the commercialization and sale of our product candidates. We may never succeed in obtaining regulatory approval for any of our product candidates.
Research and Development Expenses
Our research and development expenses for the six months ended December 31, 2020 included research and development consulting expenses and costs associated with development of our lead product candidate, ANEB-001.
We anticipate that our research and development activities will account for a significant portion of our operating expenses and these costs are expensed as incurred. Following the closing of this offering, we expect to significantly increase our research and development efforts as we continue to develop ANEB-001 and conduct clinical trials with patients suffering from symptoms of cannabinoid overdose, as well as continue to expand our product-candidate pipeline. We anticipate research and development expenses will include:
| ● | employee-related expenses, such as salaries, share-based compensation, benefits and travel expense for research and development personnel that we plan to hire; |
| 53 |
| ● | direct third-party costs such as expenses incurred under agreements with contract research organizations, or CROs, and contract manufacturing organizations, or CMOs; | |
| ● | costs associated with research and development activities of consultants; | |
| ● | manufacturing costs in connection with producing materials for use in conducting preclinical studies and clinical trials; | |
| ● | other third-party expenses directly attributable to the development of our product candidates; and | |
| ● | amortization expense for future asset purchases used in research and development activities. |
We currently have one lead product candidate; and therefore, do not track our internal research and development expenses on an indication-by-indication basis.
Research and development activities will continue to be central to our business model. Product candidates in early stages of clinical development, generally have high development costs, primarily due to multiple clinical trials, API, drug product and clinical materials manufacturing, milestone payments, IND process and clinical trial planning. We expect our research and development expenses to be significant over the next several years as we advance our current clinical development program and prepare to seek regulatory approval.
At this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the development of any product candidates that we develop from our programs. We are also unable to predict when, if ever, material net cash inflows will commence from sales of product candidates we develop, if at all. This is due to the numerous risks and uncertainties associated with developing product candidates, including the uncertainty of:
| ● | the duration, costs and timing of clinical trials of our current and future indication expansion programs and new product candidates; | |
| ● | successful completion of preclinical studies and clinical trials; | |
| ● | sufficiency of our financial and other resources to complete the necessary preclinical studies and clinical trials; | |
| ● | acceptance of INDs for our planned clinical trial or future clinical trials; | |
| ● | successful enrollment and completion of clinical trials; | |
| ● | successful data from our clinical program or future clinical programs that supports an acceptable risk-benefit profile of our product candidates in the intended populations; | |
| ● | receipt of regulatory and marketing approvals from applicable regulatory authorities; | |
| ● | receipt and maintenance of marketing approvals from applicable regulatory authorities; | |
| ● | establishing agreements with third-party manufacturers for clinical supply for our clinical program or future clinical programs and commercial manufacturing, if our product candidate is approved; | |
| ● | entry into collaborations to further the development of our product candidates; | |
| ● | obtaining, maintaining, protecting, expanding and enforcing patent and trade secret protection or regulatory exclusivity for our product candidates; and | |
| ● | successfully launching commercial sales of our product candidates if and when approved. |
A change in the outcome of any of these variables with respect to the development of any of our programs or any product candidate we develop would significantly change the costs, timing and viability associated with the development of such program or product candidate.
| 54 |
General and Administrative Expenses
General and administrative expenses for the six months ended December 31, 2020 consisted primarily of professional fees, stock-based compensation, personnel cost and rent.
We anticipate that our general and administrative expenses will increase in the future to support our continued development efforts, ongoing and future potential research and development activities, and increased costs of operating as a public company. These increased costs will likely include additional personnel, outside consultants, lawyers, and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company, including services associated with maintaining compliance with the requirements of Nasdaq and the SEC, insurance, and investor relations costs. If any of our current or future indication expansion programs or new product candidates obtains U.S. regulatory approval, we expect that we would incur significantly increased expenses associated with building a sales and marketing team.
Results of Operations
Six months ended December 31, 2020
The following table sets forth our results of operations for the six months ended December 31, 2020. As such, the results for the six months ended December 31 2020 may not provide a complete assessment of our financial performance and future periods.
| Six months ended | ||||
| December 31, 2020 | ||||
| Operating expenses: | ||||
| Research and development | $ | 190,268 | ||
| General and administrative | 386,649 | |||
| Total operating expenses | 576,917 | |||
| Other expense: | ||||
| Interest expense | (8,066 | ) | ||
| Loss from operations before taxes | (584,983 | ) | ||
| Income tax expense | - | |||
| Net loss | $ | (584,983 | ) | |
Research and Development Expenses
Research and development expenses of $190,268 for the six months ended December 31, 2020 consisted primarily of costs incurred for the research and development of our lead clinical candidate, ANEB-001, which included expenses incurred for consultants, clinical research activities, and clinical study materials.
General and Administrative Expenses
General and administrative expenses of $386,649 for the six months ended December 31, 2020 consisted primarily of professional fees, stock-based compensation, personnel costs and rent.
Interest Income (Expense), Net
Interest expense of $8,066 for the six months ended December 31, 2020 was related to two promissory notes issued to a related party.
Income Taxes
For interim periods, we estimate the annual effective income tax rate and apply the estimated rate to the year-to-date income or loss before income taxes. The effective income tax rate for the six months ended December 31, 2020 was 0.0%. Currently, we have recorded a full valuation allowance against our net deferred tax assets, primarily related to federal net operating losses and research and development credits.
| 55 |
Period from April 23, 2020 (Inception) to June 30, 2020
The following table sets forth our results of operations for the period from April 23, 2020 (date of inception) to June 30, 2020. As such, the results from April 23, 2020 (inception) to June 30, 2020 may not provide a complete assessment of our financial performance for future periods.
| For the period from April 23, 2020 (inception) | ||||
| to June 30, 2020 | ||||
| Operating expenses: | ||||
| Research and development | $ | 150,000 | ||
| General and administrative | 23,351 | |||
| Total operating expenses | 173,351 | |||
| Other expense: | ||||
| Interest expense | (1,286 | ) | ||
| Loss from operations before taxes | (174,637 | ) | ||
| Income tax expense | - | |||
| Net loss | $ | (174,637 | ) | |
Research and Development Expenses
Research and development expenses of $150,000 for the period from April 23, 2020 (inception) to June 30, 2020 represented the initial costs to secure the License Agreement.
General and Administrative Expenses
General and administrative expenses of $23,351 for the period from April 23, 2020 (inception) to June 30, 2020 were primarily related to legal fees associated with the formation of Anebulo Pharmaceuticals, Inc.
Interest Income (Expense), Net
Interest expense of $1,286 for the period from April 23, 2020 (inception) to June 30, 2020 was primarily related to two promissory notes issued to a related party.
Income Taxes
Since our inception in April 2020, we have incurred operating losses and negative cash flows from operations. At June 30, 2020, we had federal and net operating loss carryforwards of $174,637 with no expiration. In light of these considerations, as well as uncertainty as to when we might generate taxable income, we have recorded a full valuation allowance of $34,927. The amount of the net deferred tax asset considered realizable could be adjusted in the future if estimates of taxable income change or if objective negative evidence is no longer present and additional weight may be given to subjective evidence.
Liquidity and Capital Resources
Overview
To date, we have financed our operations primarily with proceeds from sales of our series A convertible preferred stock and issuance of two promissory notes to a related party. From our inception through June 30, 2020, we received gross proceeds of $3,200,000. As of December 31, 2020 and June 30, 2020, we had cash and cash equivalents of $2,480,003 and $3,024,980 and an accumulated deficit of $759,620 and $174,637, respectively. As of December 31, 2020 and June 30, 2020, we had $209,352 and $201,286 of outstanding debt, respectively. Additionally, we anticipate receiving proceeds from the issuance of milestone warrants of $2,250,000, pursuant to the Securities Purchase Agreement, by the end of the first calendar quarter of 2021.
| 56 |
We intend to use the net proceeds of this offering to make expenditures to fund proprietary research and development of our ANEB-001 product candidate and to support preclinical testing and clinical trials necessary for regulatory filings. A portion of the net proceeds of this offering may be used for the acquisition or licensing of complementary technologies, products or businesses. We also may use a portion of these proceeds to repay certain amounts of debt currently outstanding. The net proceeds of this offering will also be available for working capital and other general corporate purposes, including enhancing our corporate infrastructure and systems to assist in creating a more robust means of tracking data, automating back office functions and improving our financial reporting system. We will need additional funding to complete the clinical development of, seek regulatory approval for and commercially launch ANEB-001 and other pipeline development products.
Until such time, if ever, as we can generate substantial product revenue from sales of any of our current or future product candidates, we expect to finance our cash needs through a combination of equity offerings, debt financings and potential collaboration, license or development agreements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that could adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. Adequate additional funding may not be available to us on acceptable terms, or at all. If we are unable to raise capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of our product candidates, grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves or potentially discontinue operations.
Financing Transactions
On May 28, 2020 and June 18, 2020, we executed two promissory notes payable to Dr. Lawler in the aggregate principal amount of $200,000, reflecting cash advances by the lender to us in May and June 2020. The indebtedness is unsecured and bears interest at the rate of 8.0% per year. All accrued and unpaid interest and principal on the promissory note issued on May 28, 2020 is due and payable on demand by the holder on or after the date on which we consummate an equity financing (or series of equity financings having materially similar terms and conditions) pursuant to which we sell and issue shares of preferred stock for total aggregate gross proceeds of at least $2,500,000. As of the date of this prospectus, the related party investor has not yet demanded repayment of the note.
All accrued and unpaid interest and principal on the promissory note issued on June 18, 2020 is due and payable on demand by the holder on June 17, 2023. All accrued and unpaid interest and principal under both promissory notes shall be automatically due upon a change in control, defined generally as a consolidation or merger of our company, any transaction or series of transactions in which in excess of 50% of our voting power is transferred, a sale of all or substantially all of our assets or an exclusive license of all or substantially all our material intellectual property. We have used the proceeds of the promissory notes to fund organizational costs and expenses.
On June 18, 2020, we received gross proceeds of $3,000,000 from a private placement of our series A preferred stock (the “Private Placement”), convertible into 341,250 shares of our common stock, pursuant to the terms of a securities purchase agreement (the “Securities Purchase Agreement”) with 22NW, LP, an institutional accredited investor affiliated with Aron R. English, who became a director of our company at such time. The series A preferred stock is convertible into shares of common stock automatically upon the closing of this offering. The conversion price of the series A preferred stock is $8.7912 per share. The conversion price is subject to adjustment if, at any time prior to conversion of the shares, we issue in a financing additional shares of common stock or other equity or equity-linked securities at a purchase, conversion or exercise price less than $8.7912 per share. In any such case, we have agreed to issue additional shares of series A preferred stock to the investors so that the effective purchase price per share in the Private Placement is reduced by a weighted-average anti-dilution percentage that takes into account both the lower per share purchase, conversion or exercise price and the number of such additional shares issued at the lower price. No adjustment will be made, however, in respect of shares of common stock or stock options issued to employees, directors or consultants, or in connection with acquisitions of other corporations or strategic collaborations approved by our board of directors.
| 57 |
As part of the Private Placement, 22NW, LP and Mr. English, individually, further agreed under the Securities Purchase Agreement to purchase upon the achievement of certain corporate events “milestone” warrants for $1.95754 per warrant (or $2,250,000 in the aggregate). The warrants are exercisable for cash for up to 1,149,401 shares of series A preferred stock at an exercise price of $10.11 per share or on a “net-exercise” basis into such lesser number of shares of series A preferred stock by surrendering a portion of the underlying warrant shares, based on the positive difference between the stated warrant exercise price and the initial public offering price per share in this offering, to pay the exercise price. The warrants must be purchased upon our achievement of (i) a filing with the FDA of an investigational new drug application or the making of an analogous regulatory filing in any foreign jurisdiction, whichever is earlier, and (ii) an arrangement by us to produce the active pharmaceutical ingredient of ANEB-001 in amounts sufficient to facilitate the consummation of a trial pursuant to such regulatory filing. The milestone warrants may also be purchased at any time at the option of 22NW, LP and Mr. English.
Cash Flows
The following table sets forth a summary of our cash flows for the six months ended December 31, 2020:
| Six months ended | ||||
| December 31, 2020 | ||||
| Net cash used in operating activities | $ | (544,977 | ) | |
| Net decrease in cash and cash equivalents | $ | (544,977 | ) | |
Operating Activities
During the six months ended December 31, 2020, our operating activities used $544,977 in cash, which was less than the net loss of $584,983, primarily due to the non-cash stock-based compensation and the accrued interest on our two outstanding promissory notes, increases in accounts payable, and accrued expenses, and a decrease in a related party receivable. These charges were offset by increases in prepaid expenses and other current assets and deferred costs associated with this offering.
Outlook
Based on the expected net proceeds from this offering, our research and development plans and our timing expectations related to the development of our clinical programs, we expect that the net proceeds from this offering will enable us to fund our operating expenses, clinical development, milestone payments and capital expenditure requirements for at least the next 18 months. However, we have based this estimate on assumptions that may prove to be incorrect, and we could use our capital resources sooner than we expect.
Contractual Obligations and Commitments
License Agreement with Vernalis
On May 26, 2020, we entered into an exclusive License Agreement with Vernalis. Pursuant to the License Agreement, Vernalis granted us an exclusive worldwide royalty-bearing license to develop and commercialize a compound that we refer to as ANEB-001, as well as access to and a right of reference with respect to any regulatory materials under its control. The License Agreement allows us to sublicense the rights thereunder to any person with similar or greater financial resources and expertise without Vernalis’s prior consent, provided the proposed sublicensee is not developing or commercializing a product that contains a CB1 antagonist or is for the same indication covered by the trials or market authorization for ANEB-001. In exchange for the exclusive license, we agreed to pay Vernalis a non-refundable signature fee of $150,000, total potential developmental milestone payments of up to $29,900,000, total potential sales milestone payments of up to $35,000,000, and low to mid-single digit royalties on net sales.
| 58 |
Under the License Agreement, we purchased the API for ANEB-001 from Vernalis on an “as is” basis for $20,000. We have the sole discretion to carry out the development and commercialization of ANEB-001, including obtaining regulatory approvals, and we are responsible for all costs and expenses in connection therewith. We have access to certain regulatory materials, including study reports from clinical and non-clinical trials, under Vernalis’s control. We agreed to use commercially reasonable efforts to (i) develop and commercialize ANEB-001 in the United States and certain European countries and (ii) conduct a Phase 2 and human clinical trial within specified periods, which periods could be extended for a nominal fee. We also agreed to provide Vernalis with periodic reports of our activities and notice of market authorization within specified timeframes.
Promissory Notes
On May 28, 2020 and June 18, 2020, we executed two promissory notes payable to Dr. Lawler in the aggregate principal amount of $200,000, reflecting cash advances by the lender to us in May and June 2020. The indebtedness is unsecured and bears interest at the rate of 8.0% per year. All accrued and unpaid interest and principal on the promissory note issued on May 28, 2020 is due and payable on demand by the holder on or after the date on which we consummate an equity financing (or series of equity financings having materially similar terms and conditions) pursuant to which we sell and issue shares of preferred stock for total aggregate gross proceeds of at least $2,500,000. As of the date of this prospectus, the related party investor has not yet demanded repayment of the note.
All accrued and unpaid interest and principal on the promissory note issued on June 18, 2020 is due and payable on demand by the holder on June 17, 2023. All accrued and unpaid interest and principal under both promissory notes shall be automatically due upon a change in control, defined generally as a consolidation or merger of our company, any transaction or series of transactions in which in excess of 50% of our voting power is transferred, a sale of all or substantially all of our assets or an exclusive license of all or substantially all our material intellectual property. We have used the proceeds of the promissory notes to fund organizational costs and expenses. As of December 31, 2020 and June 30, 2020, we had $209,352 and $201,286 of outstanding debt, respectively.
Office Lease, Manufacturing Contract and CRO Contract
In August 2020, we signed a one-year lease subleasing office space from a related party. The annualized lease obligation is approximately $14,000. In October 2020, we entered into an agreement with a third-party contract manufacturing organization. The total cost for the manufacturing contracts is approximately $973,000. Subsequently in February 2021, we entered into an agreement with a third-party contract research organization (“CRO”) to manage and conduct our Phase 2 clinical trial in the fourth calendar quarter of 2021 with the anticipation of completing the trial by the first calendar quarter of 2022. The total cost for the CRO agreement is approximately €1,450,758 or $1,760,000.
We enter into contracts in the normal course of business with clinical trial sites and clinical supply manufacturers and other services and products for operating purposes. These contracts generally provide for termination after a notice period, and therefore, are cancellable contracts.
Going Concern Qualification
The financial statements and related notes included elsewhere in this prospectus have been prepared as though we will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. We have incurred operating losses and negative cash flows from operations since inception. As of December 31, 2020, we had an accumulated deficit of $759,620. Management expects to continue to incur operating losses and negative cash flows from operations in fiscal year 2021. In addition, we are subject to a series of potential development milestone payments associated with the License Agreement, with a range of payments from $350,000 to $3,000,000. We have financed our operations to date with proceeds from the sale of our series A preferred stock and issuance of the two promissory notes to a related party.
We will need to raise additional capital in order to continue to fund operations, including milestone obligations under the License Agreement. We believe we will be able to obtain additional capital through equity or convertible debt financings or other arrangements to fund operations; however, there can be no assurance that such additional financing, if available, can be obtained on acceptable terms. If we are unable to obtain such additional financing, future operations would need to be scaled back or discontinued.
| 59 |
Accordingly, these factors raise substantial doubt about our ability to continue as a going concern within one year after the date the financial statements are available to be issued. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Off-Balance Sheet Arrangements
During the six months ended December 31, 2020, we did not have any off-balance sheet arrangements as defined under SEC rules.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of these financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities as of the dates of the balance sheets and the reported amounts of expenses during the reporting periods. In accordance with U.S. GAAP, we evaluate our estimates and judgments on an ongoing basis. Significant estimates include assumptions used in the determination of some of our costs incurred under our Services Agreement and which costs are charged to research and development and general and administrative expense. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We define our critical accounting policies as those under U.S. GAAP that require us to make subjective estimates and judgments about matters that are uncertain and are likely to have a material impact on our financial condition and results of operations, as well as the specific manner in which we apply those principles. While our accounting policies are more fully described in Note 3 to our financial statements appearing elsewhere in this prospectus, we believe the following are the critical accounting policies used in the preparation of our financial statements that require significant estimates and judgments.
Accrued Research and Development Expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued research and development expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated costs incurred for the services when we have not yet been invoiced or otherwise notified of the actual costs. The majority of our service providers invoice us in arrears for services performed and some require advanced payments. We make estimates of our accrued expenses of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. Examples of estimated accrued research and development expenses include fees paid to:
| ● | CROs in connection with performing research services on our behalf and any clinical trials; | |
| ● | Investigative sites or other providers in connection with studies and any clinical trials; | |
| ● | Vendors in connection with the preparation of our NDA file, market and patient awareness programs, market research and analysis and medical education; and | |
| ● | Vendors related to product manufacturing, development and distribution of clinical supplies. |
We base our expenses for services rendered on our estimates of the services received and efforts expended pursuant to quotes, contracts and communicating with our vendors. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payments. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or amount of prepaid or accrued expenses accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in us reporting amounts that are too high or too low in any particular period.
| 60 |
Stock-based Compensation
We recognize stock-based compensation expense related to stock options granted to employees and non-employees based on the estimated fair value of the awards on the date of grant. We estimate the grant date fair value, and the resulting stock-based compensation expense, for stock options that only have service vesting requirements or performance-based vesting requirements without market conditions using the Black-Scholes option-pricing model. The grant date fair value of the stock-based awards with service vesting requirements is generally recognized on a straight-line basis over the requisite service period, which is generally the vesting period of the respective awards. Determining the appropriate amount to expense for performance-based awards based on the achievement of stated goals requires judgment. The estimate of expense is revised periodically based on the probability of achieving the required performance targets and adjustments are made as appropriate. The cumulative impact of any revisions is reflected in the period of change. If any applicable financial performance goals are not met, no compensation cost is recognized, and any previously recognized compensation cost is reversed.
The Black-Scholes option-pricing model requires the use of highly subjective assumptions, which determine the fair value of stock- based awards. These assumptions include:
Expected term - Our expected term represents the period that the stock-based awards are expected to be outstanding and is determined using the simplified method (based on the mid-point between the vesting date and the end of the contractual term). For stock-based awards granted to non-employees, the expected term represents the contractual term of the award.
Common stock price - The Board of directors estimates the fair value of common stock. Given the absence of a public trading market for its common stock, and in accordance with the American Institute of Certified Public Accountants’ Practice Guide, Valuation of Privately Held-Company Equity Securities Issued as Compensation, the board of directors exercises reasonable judgment and considers a number of objective and subjective factors to determine its best estimate of the fair value of the common stock, as further described below under “Common stock valuations.”
Expected volatility – We are a privately held company and did not have any trading history for its common stock and the expected volatility was estimated using weighted-average measures of implied volatility and the historical volatility of its peer group of companies for a period equal to the expected life of the stock options. The peer group of publicly traded biopharmaceutical companies was chosen based on their similar size, stage in the life cycle or area of specialty.
Risk-free interest rate - The risk-free interest rate is based on the rates paid on securities issued by the U.S. Treasury with a term approximating the expected life of the stock options.
Expected dividend – We have never paid, and do not anticipate paying, cash dividends on our common stock. Therefore, the expected dividend yield was assumed to be zero.
In addition to the Black-Scholes assumptions, we adopted ASU 2016-09 in June 2020. As a result, we made an entity-wide accounting policy election to account for pre-vesting award forfeitures when they occur.
In September 2020, we awarded 163,750 shares of restricted common stock to Daniel Schneeberger, our Chief Executive Officer, at a grant date fair value of $0.65 per share. The restrictions are subject to the satisfaction of certain performance targets and vesting requirements pursuant to the award and employment agreement.
As of December 31, 2020, we had not issued any stock option awards.
| 61 |
Common Stock Valuations
Prior to this offering, the fair value of our common stock was estimated on each grant date by our board of directors. In order to determine the fair value of our common stock, our board of directors considered, among other things, timely valuations of our common stock prepared by an unrelated third-party valuation firm in accordance with the guidance provided by the American Institute of Certified Public Accountants Practice Guide, Valuation of Privately Held-Company Equity Securities Issued as Compensation. Given the absence of a public trading market for our common stock, our board of directors exercised reasonable judgment and considered a number of objective and subjective factors to determine the best estimate of the fair value of our common stock, including (i) our business, financial condition and results of operations, including related industry trends affecting our operations; (ii) our forecasted operating performance and projected future cash flows; (iii) the illiquid nature of our common stock; (iv) the rights and privileges of our common stock; (v) market multiples of our most comparable public peers and (vi) market conditions affecting our industry.
After the closing of this offering, our board of directors will determine the fair value of our shares of common stock underlying stock-based awards based on the closing price of our common stock as reported by Nasdaq on the date of grant.
Income taxes
We provide for income taxes under the asset and liability method. Current income tax expense or benefit represents the amount of income taxes expected to be payable or refundable for the current year. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
When uncertain tax positions exist, we recognize the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. As of December 31, 2020, we did not have any significant uncertain tax positions.
As of June 30, 2020 and December 31, 2020, our total deferred tax assets were approximately $35,000 and $125,000, respectively. Due to our lack of earnings history and uncertainties surrounding our ability to generate future taxable income, the net deferred tax assets have been fully offset by a valuation allowance. The deferred tax assets were primarily comprised of federal and state tax net operating loss (“NOL”) carryforwards. In June 2020, we issued series A preferred stock. We may experience additional ownership changes in the future as a result of subsequent shifts in our stock ownership, including this offering, some of which may be outside of our control. If a further ownership change were to occur, our ability to use our NOL carryforward might be limited.
Recent Accounting Pronouncements
See Note 3 to Notes to Audited and Unaudited Interim Financial Statements included elsewhere in this prospectus for more information.
The JOBS Act
We are an “emerging growth company,” or EGC, as defined in the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies.
We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (i) are no longer an EGC or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period, and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
| 62 |
We will remain an EGC until the earliest of (i) the last day of our fiscal year (a) following the fifth anniversary of the completing of this offering, (b) in which we have total annual gross revenues of at least $1.07 billion or (ii) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of the prior December 31 and (iii) the date on which we have issued more than $1.0 billion in non-convertible debt securities over a three-year period.
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Fluctuation Risk
We are exposed to market risk related to changes in interest rates. As of December 31, 2020, our cash and cash equivalents consisted of cash and demand deposit accounts. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates. Because of the short-term nature of the instruments in our portfolio and the low interest rates on our interest-bearing operating accounts, an immediate 10% change in market interest rates would not have a material impact on the fair market value of our investment portfolio or on our financial position or results of operations.
As of December 31, 2020, we had $209,352 of borrowings outstanding. The two promissory notes bear simple interest at a fixed annual rate of 8.0%. An immediate 10% change in the prime rate on this borrowing level would have no material impact on our debt-related obligations, financial position or results of operations.
Foreign Currency Fluctuation Risk
We are not currently exposed to significant market risk related to changes in foreign currency exchange rates; however, we have contracted with and may continue to contract with foreign vendors that are located in Europe. Our operations may be subject to fluctuations in foreign currency exchange rates in the future.
Inflation Fluctuation Risk
Inflation generally affects us by increasing our cost of labor and clinical trial costs. We do not believe that inflation had a material effect on our business, financial condition or results of operations for the six months ended December 31, 2020.
Covid-19 Business Update
In March 2020, the World Health Organization declared the global novel coronavirus disease 2019 (Covid-19) outbreak a pandemic. As of December 31, 2020, our operations have not been significantly impacted by the Covid-19 outbreak. However, we cannot at this time predict the specific extent, duration, or full impact that the Covid-19 outbreak will have on our financial condition and operations, including ongoing and planned clinical trials.
| 63 |
Overview
We are a clinical-stage biotechnology company developing novel solutions for people suffering from cannabinoid overdose and substance addiction. Our lead product candidate, ANEB-001, is intended to reverse the negative effects of cannabinoid overdose within 1 hour of administration. The signs and symptoms of cannabinoid overdose range from profound sedation to anxiety and panic to psychosis with hallucinations. There is no approved medical treatment currently available to specifically alleviate the symptoms of cannabinoid overdose. If approved by the FDA, we believe ANEB-001 has the potential to be the first FDA approved treatment of its kind on the market for reversing the effects of THC, the principal psychoactive constituent of cannabis. Clinical trials completed to date have shown that ANEB-001 is rapidly absorbed, well tolerated and leads to weight loss, an effect that is consistent with central CB1 antagonism. We intend to launch a Phase 2 proof - of - concept trial for cannabinoid overdose in the fourth quarter of 2021.
Cannabinoid overdoses have become a widespread health issue in the United States, particularly in the increasing number of states that have legalized cannabis for personal and recreational use. The ingestion of large quantities of THC is a major cause of cannabinoid overdose. Excessive ingestion of THC via edible products such as candies and brownies, and overdoses of synthetic cannabinoids (also known as “synthetics,” “K2” or “spice”), are two leading causes of THC-related emergency room visits. Synthetic cannabinoids are analogous to fentanyl for opioids insofar as they are more potent at the cannabinoid receptor than their natural product congener THC.
In recent years, hospital emergency rooms across the United States have seen a dramatic increase in patient visits with cannabis-related conditions. Before the legalization of cannabis, an estimated 450,000 patients visited hospital emergency rooms for cannabis-related conditions. In 2014, this number more than doubled to an estimated 1.1 million patients, according to data published in “Trends and Related Factors of Cannabis-Associated Emergency Department Visits in the United States: 2006-2014,” Journal of Addiction Medicine (May/June 2019), which provided a national estimate analyzing data from The Nationwide Emergency Department Sample (“NEDS”), the largest database of U.S. hospital-owned emergency department visits. Based on our own analysis of the most recent NEDS data, we believe that the number of hospitalizations grew to 1.74 million patients in 2018 and was growing at an approximately 15% compounded annual growth rate between 2012 and 2018. We believe the number of cannabis-related hospitalizations and other health problems associated with cannabinoid overdoses such as depression, anxiety and mental disorders will continue to increase substantially as more states pass laws legalizing cannabis for medical and recreational use. Given the consequences, there is an urgent need for a treatment to rapidly reverse the symptoms of cannabinoid overdose.
Our Lead Product Candidate
Our objective is to develop and commercialize new treatments options for patients suffering from addiction. Our lead product candidate is ANEB-001, a potent, small molecule cannabinoid receptor antagonist, to address the unmet medical need for a specific antidote for cannabinoid overdose. ANEB-001 is an orally bioavailable, rapidly absorbed treatment that we anticipate will reverse the symptoms of cannabinoid overdoses, in most cases within 1 hour of administration. Our proprietary position in the treatment of cannabinoid overdose is protected by rights to two patent applications covering various methods of use of the compound and delivery systems. We anticipate starting our first Phase 2 trial for ANEB-001 in the fourth quarter of 2021.
Cannabinoids are a class of chemical compounds that are naturally occurring and are primarily found in cannabis plant extracts. The two major cannabinoids found in cannabis plant extracts include THC and CBD. These compounds bind themselves to CB1 and CB2 cannabinoid receptors, which are found throughout the body. Specifically, CB1 receptors are concentrated in the brain and central nervous system, while CB2 receptors are found mostly in peripheral organs and are associated with the immune system. When the chemical compounds bind themselves to these cannabinoid receptors, the process elicits certain physiological responses. Physiological responses to cannabinoids may vary among individuals. Some of the effects of cannabinoids have been shown to impact nervous system functions, immune responses, muscular motor functions, gastrointestinal maintenance, blood sugar management, and the integrity of ocular functions.
| 64 |
Individuals can use or consume cannabinoids in natural or unnatural formulations, orally or by inhalation, and intentionally and unintentionally, all of which can result in an overdose. Natural formulations include edibles and marijuana cigarettes and unnatural formulations include synthetics. Individuals consume cannabinoids orally by ingesting edibles or synthetics and by inhalation through smoking marijuana cigarettes or synthetics. Cannabinoids can also be ingested unintentionally through these same methods where, for example, children consume edibles by mistaking them for common consumer items like candy that would not otherwise contain THC. Symptoms of cannabinoid overdoses produced by edibles and synthetics can include psychosis, panic and anxiety, feelings of paranoia, agitation, hallucinations, nausea, vomiting, cardiac arrhythmias, seizures and death. Many of these symptoms can require emergency medical attention and can take hours to days to resolve depending on the particular product and amount ingested. Currently, there is no specific treatment to reverse cannabis overdose and physicians have to rely on supportive care, including benzodiazepines, and wait for the body to metabolize the THC or synthetic cannabinoid.
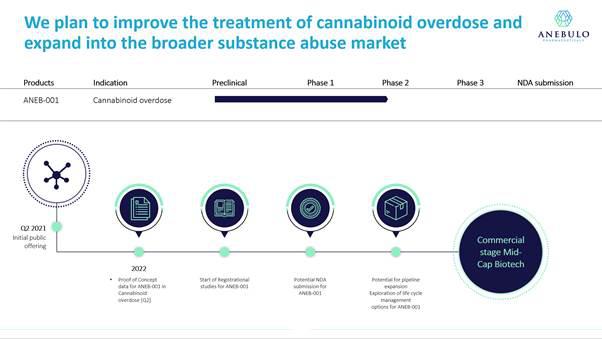
| 65 |
Our Market Opportunity
Cannabinoid overdoses have become a widespread health issue in the United States as an increasing number of states have legalized cannabis for personal and recreational use. As of December 31, 2020, cannabis was legal for recreational use in 15 states and legal for medical use in 35 states. Additionally, the Centers for Disease Control and Prevention and recent news reports have described how the stress, anxiety and depression from the prolonged stay-at-home conditions surrounding the Covid-19 pandemic appears to be resulting in excessive drug and cannabis use by individuals, whether in jurisdictions where such use is legal or not.
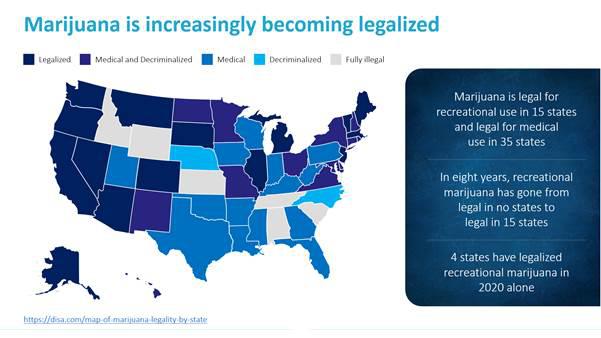
Cannabinoid overdoses frequently occur due to the ingestion of edibles, which can contain relatively large amounts of THC, and consumption of synthetics. Symptoms of cannabinoid overdoses produced by edibles and synthetics can include psychosis, panic and anxiety, feelings of paranoia, agitation, hallucinations, nausea, vomiting, cardiac arrhythmias, seizures and death. These symptoms can require emergency medical attention and can take hours to days to resolve. According to an article published in the Journal of Addiction Medicine that analyzed data from NEDS, an estimated 1.1 million emergency department visits were associated with cannabis in 2014. We have performed our own independent analysis of all currently available NEDS datasets and estimated that the number of cannabis-associated emergency department visits increased to 1.74 million patients in 2018. The number of cannabis-associated emergency department visits has grown at a 15% compounded annual growth rate from 2012 to 2018, which is when states first began legalizing recreational cannabis use.
| 66 |
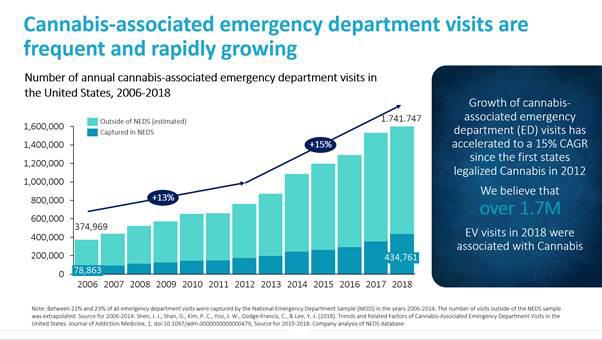
Source for 2006-2014: Shen, J. J., Shan, G., Kim, P. C., Yoo, J. W., Dodge-Francis, C., & Lee, Y.-J. (2018). Trends and Related Factors of Cannabis-Associated Emergency Department Visits in the United States. Journal of Addiction Medicine, 1. doi:10.1097/adm.0000000000000479, Source for 2015-2018: Company analysis of NEDS database.
We believe that both the number of cannabis-associated emergency department visits and the unmet medical need will continue to grow due to the increasing availability and consumption of edibles. In THC-containing edibles, the median dose of THC can be many times more potent than the recommended safe dosage and as much as 8 times more potent than a rolled marijuana cigarette. Edibles are frequently manufactured as common consumer products, such as brownies, cookies, candies and gummy snacks with brightly-colored packaging. THC concentrations in edibles peak after a delay of about 2 to 4 hours from ingestion. This contrasts with smoking cannabis, which causes THC concentrations to peak in about 3 to 10 minutes from inhalation. Consumers are likely to approach edibles with the same serving size expectations as consumer products without THC. Moreover, children are particularly at risk for accidentally consuming edibles due to their brightly-colored packaging and formulation into candies and sweets. The confluence of these factors can be dangerous and increases the risk of cannabinoid overdose. Emergency department visits were 33 times more likely for edibles as compared with other routes of cannabis consumption, according to the recent article “Mental Health-related Emergency Department Visits Associated with Cannabis in Colorado,” published in Academic Emergency Medicine (May 2018). Sales of edibles are rapidly growing, according to data collected by Statista, and are expected to continue growing for the foreseeable future.
In November 2020, we engaged a consultation and data services provider to conduct a survey of U.S. physicians concerning patient emergency room visits for cannabinoid overdoses within the past 12 months. Based on its survey of 27 emergency room physicians throughout the United States, the survey provider reported that the surveyed physicians saw on average 10.5 patients (a range of 2 to 45 patients) with cannabis intoxication per month. The survey asked these physicians to rank on a scale of 1 to 10 (i) the need for a cannabinoid antagonist to treat cannabis intoxication; (ii) the likelihood of their prescribing a cannabinoid antagonist that reverses cannabis intoxication within 30 minutes of administration; and (iii) the likelihood of such cannabinoid antagonist reducing the need for supportive medication to manage certain cannabis intoxication symptoms, such as agitation and acute psychosis. In response to these questions, the surveyed physicians ranked the need for a cannabinoid antagonist at an average of 7.52 out of 10, the likelihood of prescribing a cannabinoid antagonist that reverses cannabis intoxication within 30 minutes of administration at an average of 7.44 out of 10, and the likelihood of a specific cannabinoid antagonist reducing the need for supportive medication to manage certain cannabinoid overdose symptoms at an average of 7.48 out of 10.
| 67 |
We believe that the market opportunity for our lead product candidate, ANEB-001, will continue to expand and accelerate if additional states pass laws to legalize recreational cannabis use. On December 4, 2020, the U.S. House of Representatives voted in favor of a bill to decriminalize marijuana at the federal level by removing cannabis from the list of controlled substances under the Controlled Substances Act. Although it is currently uncertain whether this bill will be subsequently approved by the U.S. Senate and signed into law by the President, in the event the use of cannabis is legalized in the United States at the federal level, we believe that the greater anticipated number of users will significantly increase the potential need for our lead candidate.
We believe that overdose due to synthetic cannabinoids is an area with particularly high unmet medical need. Synthetics are among the fastest growing class of psychoactive drugs worldwide and can be as much as 85 times as potent as THC. Unlike edibles and other cannabis products, synthetics have low shipping weights and can more readily evade traditional drug screening methods. This likely reflects the structural promiscuity of the CB1 receptor. In addition, the negative effects of an overdose from synthetics can be longer lasting and more severe when compared with THC. These negative effects could include seizures, and even death.
Our Growth Strategy
Our goal is to create a therapeutic to treat the symptoms of cannabinoid overdose and substance addiction. As noted above, there are currently no FDA approved medical treatments on the market to specifically alleviate the negative psychological effects of cannabinoid overdose. The absence and growing unmet need for such a treatment gives us the unique opportunity to create a novel solution and become a leader in the cannabinoid treatment space. To achieve our goal, our strategy will be guided by the following principles:
| ● | Develop and commercialize our ANEB-001 antagonist in the United States. We anticipate commencing our Phase 2 proof-of-concept study in the fourth quarter of 2021. We believe the data from this study may facilitate discussions of a regulatory path for ANEB-001 in the United States. | |
| ● | Explore strategic collaborations to commercialize ANEB-001. Our plan is to widely commercialize ANEB-001. To accomplish this objective, we may partner with companies that possess a direct sales force and sales representatives. | |
| ● | Strive for capital efficiency in developing ANEB-001. We aim to be capital efficient in our development of ANEB-001 by outsourcing our clinical research and data management. We anticipate this will lower our clinical development costs and improve our ability to efficiently commercialize ANEB-001 once approved by the FDA. | |
| ● | Introduce promising product candidate extensions. We are in the initial stages of introducing a non-oral formulation of ANEB-001 with the same API that we intend to develop for the use in cannabinoid hyperemesis syndrome (CHS), which is a condition that can develop following long-term use of marijuana and is characterized by cyclical episodes of nausea and vomiting that are not usually responsive to standard care. We believe that antagonizing the paradox emetogenic action of THC at the receptor and helping patients abstain from THC represent the most promising and causal treatment for CHS. | |
| ● | Develop future product candidates to treat substance-related addiction. We intend to leverage our expertise in the endocannabinoid system to develop additional product candidates for the treatment of substance addiction. CB1 antagonists have been shown to be promising in treating substance-related addiction. We believe that there is a large and growing unmet medical need for new treatment options because of the opioid and methamphetamine epidemic. |
Our Clinical Trials and Milestones
We are developing ANEB-001 to quickly and effectively combat the symptoms of cannabinoid overdose.
Preclinical Data
The preclinical characterisation of ANEB-001 was performed at Vernalis’ internal laboratory in the United Kingdom between 2003 and 2006. The compound was tested as a displacer in established radioligand binding assays for the CB1 receptor. ANEB-001 displaced the antagonist radioligand, [3H]-SR141716A from the human CB1 receptor with high affinity (0.55 nM) and was shown to be a competitive antagonist in cAMP assays. In vitro testing as a displacer in 90 binding assays and 19 enzyme and functional assays, showed that ANEB-001 had >1000x selectivity with the human CB1 receptor over all other tested receptors. Further, Vernalis demonstrated that oral administration of ANEB-001 reduced hypolocomotion in mice after 30 minutes, effectively reversing the action of THC. C57 mice administered THC 3 mg/kg in 10 minutes pre-test exhibited reduced locomotor activity when placed in automated locomotor activity cages for 15 minutes. V24343 given orally at a dose of 30 mg/kg 30 minutes pre-test significantly revered the action of THC on the total activity time parameter (p<0.01 by one way ANOVA and Newman Keuls test, n=7 per group).
In 2006 and 2007, two Phase 1 studies for the treatment of obesity were conducted by Vernalis for ANEB-001.
Phase 1a
The Phase 1a study (V24343-1Ob-01) administered single (Part A) and multiple (Part B) ascending doses of ANEB-001 for up to 14 days in otherwise healthy overweight and mildly obese subjects.
| ● | Part A randomized 18 healthy volunteers to receive either a placebo (n=18) or two single oral doses of ANEB-001, with doses ranging from 1 mg to 200 mg. No severe adverse events were observed in either group in Part A. There was no difference between treatment groups in Part A in overall incidence, number of or severity of adverse events. Probable drug-related events in the treatment arm were nausea (22%), dizziness (11%), hiccups (8%), and decreased appetite (8%). |
| 68 |
| ● | Part B randomized 32 obese volunteers to receive either a placebo (8 obese volunteers) or 4 different doses of ANEB-001 for 14 days (24 obese volunteers). No severe adverse events were observed in either group in Part B, but an increased number of mild and moderate adverse events was observed in the obese volunteers who received the 2 higher dose arms (200/50 mg and 100 mg). The observed adverse events included nausea, vomiting, diarrhea, dizziness, hiccups, decreased appetite, hyperhidrosis and feeling hot. We believe these adverse events are “on-target,” meaning they reflect CB1 antagonism, because these adverse events have also been observed with other CB1 antagonists. |
Pharmacokinetic measurements in Part A of the Phase 1a study demonstrated that ANEB-001 was rapidly absorbed by the body following oral administration and achieved blood concentrations anticipated to exceed those necessary to block the cannabinoid receptor (as indicated by the red line in the diagram below)

Vernalis also measured the impact of ANEB-001 on anxiety and depression in Part B of the Phase 1a study. Vernalis measured anxiety by using the Spielberger state score, a commonly used measure of trait and state anxiety. Vernalis found no significant impact on anxiety, except for the 200/50 mg arm, which showed increased anxiety at all assessment times. The change was driven by a single subject and may be explained by somatic adverse events, which contributed to the Spielberger score. For depression, HAMD21 was used and small increases were noted in the 75/15 mg and 200/50 mg dose, which we believe were likely driven by somatic symptoms.
Summarizing the results from the Phase 1a study, ANEB-001 doses between 1 mg and 150 mg were found to be very well tolerated in both single and multiple doses with an adverse events profile similar to placebo. There was no observed effect on the cardiovascular system, ECGs, labs or physical exams and no significant effects on anxiety or depression scores.
| 69 |
With regard to pharmacodynamics, a marked reduction in test meal energy intake was seen even at the lowest dose level in Phase 1a Part B (p<0.01 on Day 14 for OD 100mg, p<0.05 on Day 7 for OD 100mg, not statistically significant for all other cohorts). Further, Vernalis observed statistically significant decreases in body weight (p<0.001 on Day 14 for OD 100mg, p<0.05 for OD 50/5mg and OD 200/50mg, not significant for OD75/15mg) indicating that ANEB-001 was able to cross the blood-brain barrier and antagonize central cannabinoid receptors.
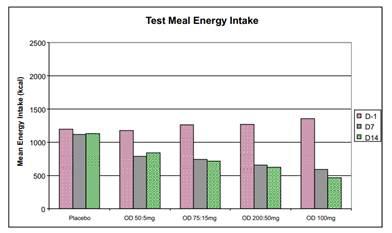
Phase 1b
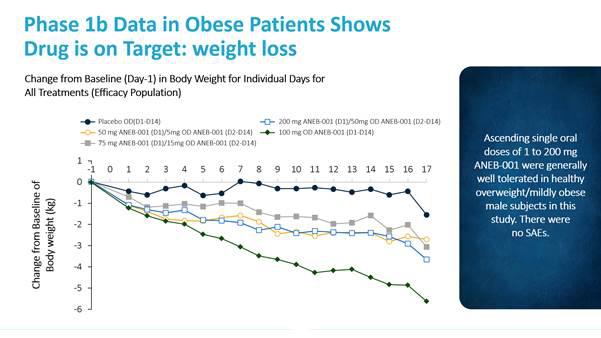
The Phase 1b study (V24343-1Ob-02) compared the pharmacokinetics of a single oral dose (1 to 200 mg) of ANEB-001 to subjects in fed and fasted states, and to subjects that were lean and overweight. There were no apparent differences in the tolerability of ANEB-001 between the subjects that were in fed and fasted states or subjects that were lean and overweight. Total AUC (or area under the curve) was approximately 30% higher in subjects in the fed state compared to the subjects in the fasted state, with similar systemic exposure for the lean and overweight subjects.
| 70 |
The results of the Phase 1 studies demonstrate that ANEB-001 was well-tolerated among healthy and obese subjects. There were no serious adverse events. The most commonly reported adverse event was gastrointestinal discomfort, which also occurred in subjects that were administered placebos. Based on the promising results of the Phase 1 studies, we believe ANEB-001 may offer the following clinical and product benefits:
| ● | Oral bioavailability. ANEB-001 will be available as an oral treatment in the form of a pill, capsule or tablet. | |
| ● | Rapid absorption. We believe ANEB-001 can rapidly reverse the signs and symptoms of cannabinoid overdose in as little as 1 hour. | |
| ● | Low likelihood of drug-to-drug interactions. Preclinical testing demonstrated that ANEB-001 did not inhibit the metabolic enzymes cytochromes 1A2, 2C9, 2C19, 2D6 and 3A4 at pharmacologically relevant concentrations. | |
| ● | Better treatment option. As an orally administered treatment tested to work in as little as 1 hour, ANEB-001 has the potential to be faster acting than intravenous (IV) treatments that may be developed by competitors. ANEB-001 has the potential to be the first treatment of its kind as there are no approved treatments currently available to specifically reverse the symptoms of cannabinoid overdose. | |
| ● | No serious adverse events. A single dose of the drug is unlikely to produce adverse events associated with chronic dosing. The most commonly reported adverse effect in our Phase 1 study was gastrointestinal discomfort, which also occurred in subjects who were administered a placebo. |
We plan to commence a Phase 2 proof - of - concept study in the fourth calendar quarter of 2021 at a center in the Netherlands to test the efficacy of a single dose of ANEB-001 on a population of approximately 100 human subjects who have been administered 10 milligrams of THC that will then be randomized to receive a placebo, low dose, medium dose or high dose of ANEB-001. We anticipate completing the Phase 2 study within approximately six months after commencing the study and having data potentially available in the first half of 2022. We believe this study will lay the foundation for us to engage with the FDA and/or comparable foreign regulatory authorities, file IND with the FDA in the United States and conduct more extensive clinical trials with the goal of generating additional clinical data that will ultimately enable us to file a marketing application with the FDA.
We have engaged contract research organizations (“CROs”) to assist us with conducting clinical trials and to provide us with consulting and development services in the various phases of the drug development process. We currently have a consultancy agreement with Traxeus Pharma Services Limited (“Traxeus”), which we entered into on July 15, 2020 (the “Consultancy Agreement”). Pursuant to the Consultancy Agreement, Traxeus provides certain pharmaceutical development services and deliverables to us in relation to the retest of an existing batch of drug substance. These services include the manufacturing and testing of a demonstration batch of the drug substance and the completion of formulation and process development for the drug product. Under the Consultancy Agreement, Traxeus is permitted to provide services to third parties that are not directly competitive to us and we are permitted to engage other CROs. The Consultancy Agreement can be terminated immediately by either party if a material breach is committed and not remedied within 60 days or a party is unable to carry on business, becomes insolvent or is subject to similar processes in any jurisdiction. In addition, we may terminate any statement of work arising under the Consultancy Agreement by providing Traxeus at least 30 days’ written notice. We plan to continue to engage CROs like Traxeus and other pharmaceutical services providers to assist us with clinical trials, the development of our lead product candidate ANEB-001.
| 71 |
Vernalis License Agreement
On May 26, 2020, we entered into an exclusive license agreement (the “License Agreement”) with Vernalis (R&D) Limited (“Vernalis”). Pursuant to the License Agreement, Vernalis granted us an exclusive worldwide royalty-bearing license to develop and commercialize a compound that we refer to as ANEB-001, as well as access to and a right of reference with respect to any regulatory materials under its control. The License Agreement allows us to sublicense the rights thereunder to any person with similar or greater financial resources and expertise without Vernalis’s prior consent, provided the proposed sublicensee is not developing or commercializing a product that contains a CB1 antagonist or is for the same indication covered by the trials or market authorization for ANEB-001. In exchange for the exclusive license, we agreed to pay Vernalis a non-refundable signature fee of $150,000, total potential developmental milestone payments of up to $29,900,000, total potential sales milestone payments of up to $35,000,000, and low to mid-single digit royalties on net sales.
Under the License Agreement, we purchased the API for ANEB-001 from Vernalis on an “as is” basis for $20,000. We have the sole discretion to carry out the development and commercialization of ANEB-001, including obtaining regulatory approvals, and we are responsible for all costs and expenses in connection therewith. We have access to certain regulatory materials, including study reports from clinical and non-clinical trials, under Vernalis’s control. We agreed to use commercially reasonable efforts to (i) develop and commercialize ANEB-001 in the United States and certain European countries and (ii) conduct a Phase 2 and human clinical trial within specified periods, which periods could be extended for a nominal fee. We also agreed to provide Vernalis with periodic reports of our activities and notice of market authorization within specified timeframes.
With respect to intellectual property, both parties agreed to retain sole ownership over their respective intellectual property as of the date of the License Agreement. In addition, we retain the sole right over certain patent rights (including patent applications) and know-how controlled by us that are necessary or reasonably useful to developing and commercializing ANEB-001 during the term of the License Agreement.
| 72 |
The License Agreement continues for an indefinite term unless and until it is terminated or until such time as all royalties and other sums cease to be payable thereunder. Our obligations to pay royalties commence upon the first commercial sale of our product and cease upon the later to occur of: (i) the tenth anniversary of the first commercial sale of our product, or (ii) the expiration date of the regulatory exclusivity of our product. We may terminate the License Agreement in its entirety at any time by providing 60 days’ prior notice to Vernalis. Moreover, a party may terminate the License Agreement for cause (i) upon written notice when the other party commits a material breach not remedied within the specified timeframes and defaults on its obligations thereunder, or (ii) when the other party is insolvent as more particularly described therein. In the event of termination, all rights and licenses granted by Vernalis will revert immediately to Vernalis; all outstanding sums as of the termination date will be immediately due and payable to Vernalis; and we will return or destroy, at Vernalis’s request, any regulatory materials, information pertaining to ANEB-001, and any unused API purchased from Vernalis. If Vernalis terminates the License Agreement due to our material breach or insolvency, or if we terminate the License Agreement at will, both parties will negotiate in good faith to grant Vernalis a license to such intellectual property and regulatory materials needed to develop and commercialize ANEB-001 and provide appropriate compensation to us within six months of the termination date.
Competition
The clinical biotechnology industry is a competitive industry characterized by technological innovation and growth. Our competitors include other biotechnology and pharmaceutical companies, academic institutions, and public and private research institutions. These entities engage in efforts to research, discover and develop new medicines and treatments for substance use. These entities also seek patent protection and licensing revenues for their research results and may compete with us in recruiting skilled talent. Some of these entities are larger and better funded than us. Our management can make no assurances that we can effectively compete with these competitors. Potential current competitors include Opiant Pharmaceuticals, Inc., which is developing a drinabant injection to treat cannabinoid overdose, and Aelis Farma, which is developing a medication based on a pregnanolone derivative to treat cannabis use disorders.
Research and Development
We are making, and expect to continue to make, substantial expenditures to fund proprietary research and development of our ANEB-001 product candidate and to support preclinical testing and clinical trials necessary for regulatory filings. Our research and development team, including a third-party contract research organization, is continuously undertaking efforts to advance research and development goals. During the period from April 23, 2020 (date of inception) to June 30, 2020 and the six months ended December 31, 2020, we incurred research and development expenses of $150,000 and $190,268, respectively.
Regulation
Government Regulation and Product Approval
We operate in an extensively regulated industry. Governmental authorities at all levels in the United States and in other countries regulate aspects of bringing therapeutics, drugs, and other biologics to market, including research, testing, safety, product approval, development, manufacture, efficacy, quality control, packaging, storage, record-keeping, promotion, labeling, advertising, marketing, distribution, sales, imports and exports of our products.
Under the Controlled Substances Act (the “CSA”), cannabis is currently considered a Schedule I controlled substance and is, therefore, illegal under federal law. A Schedule I controlled substance is defined as a drug or substance that has a high potential for abuse, has no currently accepted medical use in the United States, and lacks accepted safety for use under medical supervision. Although an increasing number of states have legalized cannabis under state laws, the use, possession and cultivation of cannabis remains a violation under federal law. The United States Supreme Court has upheld the federal government’s right to regulate and criminalize cannabis, even for medicinal uses. Federal law criminalizing the use of cannabis preempts contrary or conflicting state laws. As a result, if the federal government enforces the CSA in states that have legalized cannabis for medicinal and/or recreational uses, individuals that are charged with distributing, possessing with intent to distribute or cultivating cannabis could be subject to fines and/or terms of imprisonment. The maximum penalty is life imprisonment and a $50 million fine.
As therapeutic product for human use, ANEB-001 will be subject to regulation in the United States by the FDA under the Federal Food, Drug and Cosmetic Act (“FDCA”) and similar regulatory requirements in other countries. Regulatory requirements include, among other things, rigorous preclinical and clinical testing. The processes for commercializing our product, obtaining regulatory approval and maintaining compliance with applicable statutes and regulations require the substantial expenditure of time and financial resources and play a significant role in our research and development, production, and marketing activities. Failure to comply with these regulatory processes and other requirements could delay our ability to receive regulatory approvals, adversely affect the commercialization of our product, and hinder our ability to receive royalties or revenues.
| 73 |
In the United States, the FDA regulates drugs under the FDCA and its implementing regulations. Failure to comply with such regulations during and after the product development and approval process could result in administrative or judicial sanctions. Such sanctions include the FDA’s refusal to approve pending applications, withdrawal of an approval, placement a clinical hold, untitled or warning letters, product recalls, seizure of products, partial or complete suspension of production or distribution, injunctions, fines, refusal of government contracts, restitution, disgorgement, civil penalties and criminal penalties. The FDA generally requires the following before a drug can be marketed in the United States:
| ● | Completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices regulations; | |
| ● | Submission of an Investigational New Drug Application (“IND”), which must become effective before the commencement of human clinical studies; | |
| ● | Approval by an independent internal review board (“IRB”), at each clinical site before the initiation of each trial; | |
| ● | Performance of adequate and well-controlled human clinical studies according to Good Clinical Practice (“GCP”) regulations, to establish the safety and efficacy of the proposed drug for its intended use; | |
| ● | Preparation and submission of a New Drug Application (“NDA”); | |
| ● | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the product, or its components, are produced to ensure compliance with current Good Manufacturing Practice (“CGMP”) regulations and to ensure that the facilities, methods, and controls are adequate to preserve the drug’s identity, strength, quality, and purity; and | |
| ● | FDA review and approval of the NDA. |
Given that the testing and approval process requires a substantial commitment of time, effort and financial resources, we cannot ensure that our product will be granted approval on a timely basis.
As part of the IND, an IND sponsor must submit the preclinical test results, along with manufacturing information, analytical data and any available clinical data or literature, to the FDA. The sponsor must also include a protocol detailing the objectives of the initial clinical study, the parameters for monitoring safety, and the effectiveness criteria to be assessed (among other things) if the initial clinical study lends itself to an efficacy evaluation. Some preclinical testing may continue after submission of the IND. The IND becomes automatically effective 30 days after receipt by the FDA, unless the FDA raises questions or concerns in response to a proposed clinical study and places the study on a clinical hold within the 30-day timeframe. In such a case, the IND sponsor and the FDA must resolve any outstanding issues before commencing the clinical study. The FDA may impose clinical holds due to safety concerns or non-compliance on all product candidates within a certain pharmaceutical class at any time before or during clinical studies. In addition, the FDA can impose partial clinical holds prohibiting the initiation of clinical studies for a certain dose or of a certain duration.
In accordance with GCP regulations, all clinical studies must be conducted under the supervision of one or more qualified investigators. These regulations require informed consent in writing from all research subjects before their participation in any clinical study. An IRB must review and approve the plan for any clinical study before it commences at any institution, and the IRB must continuously review and re-approve the study at least annually. Among other things, the IRB considers whether the risks to individual participants in the clinical study are minimal and reasonable in relation to the anticipated benefits. The IRB also approves the information regarding the clinical study and the consent form that must be given to each clinical study subject or his or her legal representative. The IRB must also monitor the clinical study until completed. Each new clinical protocol and any amendments thereto must be submitted to the FDA for review, and to the IRB for approval. The protocols detail the objectives of the clinical study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety (among other things). Study sites are subject to inspection for compliance with GCP.
| 74 |
Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, for public dissemination on the ClinicalTrials.gov website.
Human clinical studies are typically conducted in three sequential phases that may overlap or be combined:
| ● | Phase 1. In Phase 1, the product is initially introduced to a limited number of healthy human subjects or patients and is tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion and, if possible, to gain early evidence on effectiveness. In the case of certain products intended to treat severe or life-threatening diseases, particularly when the product is suspected or known to be unavoidably toxic, initial human testing may be conducted in patients. | |
| ● | Phase 2. Phase 2 involves clinical studies in a limited patient population to identify potential adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific diseases and to determine dosage tolerance, optimal dosage and schedule. | |
| ● | Phase 3. In Phase 3, clinical studies are conducted on a larger patient population located in geographically dispersed clinical sites to further evaluate the dosage, clinical efficacy and safety of the product. Phase 3 clinical studies are intended to determine the overall risks and benefits of the product and provide an adequate basis for product labeling. |
Progress reports explaining the results of the clinical studies must be submitted to the FDA at least annually. Safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse events. There is no guarantee that Phase 1, Phase 2 and Phase 3 testing will be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend or terminate a clinical study at any time for various reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Likewise, an IRB can suspend or terminate approval of a clinical study at its institution if the clinical study is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
U.S. Review and Approval Processes
Upon the successful completion of the required clinical testing, an NDA is submitted to the FDA requesting approval to market the product. The NDA reports the results of product development, preclinical and clinical studies, descriptions of the manufacturing process, analytical tests conducted on the drug, proposed labeling and other relevant information.
In connection with the submission of an NDA, the payment of a substantial application user fee is required (although a waiver is available under limited circumstances, including, for the first human drug application submitted by a small business or its affiliate). The sponsor of an approved NDA is also required to pay annual program user fees.
Under the Pediatric Research Equity Act of 2003, an NDA application (or supplements thereto) for a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration must contain adequate data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective, unless the applicant has obtained a waiver or deferral.
In 2012, the Food and Drug Administration Safety and Innovation Act amended the FDCA to require submission of an initial Pediatric Study Plan (“PSP”) for any sponsor that plans to submit a marketing application for a drug that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. The initial PSP must be submitted within sixty days of an End-of-Phase 2 meeting or as may be agreed between the sponsor and the FDA. The initial PSP must contain an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA may grant deferrals for submission of data or full or partial waivers on its own volition or at the applicant’s request. The FDA and the sponsor must agree on the PSP. A sponsor can amend an initial PSP at any time (even if initially agreed upon) if changes to the pediatric plan must be considered based on data collected from preclinical studies, early phase clinical studies, and/or other clinical development programs.
| 75 |
The FDA may also require a Risk Evaluation and Mitigation Strategy (“REMS”) to mitigate any identified or suspected serious risks. The REMS typically includes risk minimization tools, medication guides, assessment plans, physician communication plans, and elements to ensure safe use, including restricted distribution methods, and patient registries.
The FDA reviews all NDA’s submitted to ensure they are sufficiently complete for substantive review before it accepts them for filing. Rather than accept an application for filing, the FDA may request additional information. In such a case, an applicant must re-submit the application along with the additional information, which remains subject to further FDA review. Once an application is accepted for filing, the FDA performs an in-depth substantive review to determine whether the product is safe and effective for its intended use.
The FDA may refer the NDA to an advisory committee consisting of experts for review, evaluation and recommendation regarding its approval and any conditions that may apply thereto. The FDA, while not bound by the recommendation of an advisory committee, considers such recommendations when making decisions. Before approving an NDA, the FDA will also inspect one or more clinical sites to ensure clinical data supporting the submission comply with GCP.
The FDA may refuse to approve an NDA if regulatory requirements are not satisfied or additional clinical data and information is required. Even after such data and information is furnished, the FDA may refuse to approve an NDA for failure to satisfy regulatory requirements. Data from clinical studies may not always be conclusive. Moreover, the FDA may disagree with the applicant’s interpretation of the data.
After evaluating an application, the FDA may issue an approval letter or a complete response letter indicating completion of the review cycle. A complete response letter typically sets forth specific conditions that must be satisfied to secure final approval of the application and may require additional clinical or preclinical testing for the FDA to reconsider the application. The FDA may identify minor deficiencies, such as requiring labeling changes, or major deficiencies, such as requiring additional clinical studies. The complete response letter may also recommend actions to ready the application for approval. An applicant can respond to a complete response letter by correcting all deficiencies and re-submitting the application, withdrawing the application or requesting a hearing.
Even after additional information is submitted, the FDA may determine that an application does not satisfy regulatory requirements and reject it. Once all conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter authorizing commercial marketing of the drug with specific prescribing information for specific indications.
Even after regulatory approval is obtained, approval may be restricted to specific diseases and dosages or limited indications for use. Such limitations could affect the commercial value of the product. On the product labeling, the FDA may require certain contraindications, warnings or precautions. In addition, the FDA may require post-approval studies, including Phase 4 clinical studies, to further evaluate safety and effectiveness. The FDA may also require testing and surveillance programs to monitor the safety of approved commercialized products. After approval, certain changes to the approved product remain subject to additional testing requirements, FDA review and approval. Such changes to the approved product include adding new indications, manufacturing changes, and additional labeling claims.
Abbreviated New Drug Applications (“ANDAs”)
Most drug products receive FDA marketing approval pursuant to an NDA for innovator products, or an ANDA for generic products. The Hatch-Waxman amendments to the FDCA established a statutory procedure for submission and FDA review and approval of ANDA’s for generic versions of branded drugs previously approved or listed by the FDA. Because brand companies (otherwise known as “innovators”) have already demonstrated the safety and efficacy of listed drugs, the FDA does not require the same demonstration for generic products. Nevertheless, the FDA requires the manufacturer of generic drugs to perform bioequivalence studies of its test product against the listed drug. The bioequivalence studies for orally administered, systemically available drug products evaluate the rate and extent to which the active pharmaceutical ingredient is absorbed into the bloodstream from the drug product and becomes available at the site of action. Bioequivalence is achieved when there is no significant difference in the rate and extent for absorption of the generic product and the listed drug. An ANDA must contain chemistry, manufacturing, labeling and stability data as well as patent certifications.
| 76 |
Approved products manufactured or distributed in accordance with the FDA regulatory process remain subject to continuing FDA oversight post-approval. Continuing regulatory requirements include periodic reporting, record-keeping, product sampling, product distribution, and advertising and reporting on adverse experiences, deviations, and other issues with the product. In addition, most post-approval changes to the approved product, including adding new indications or other labeling claims, remain subject to prior FDA review and approval. There are also continuing obligations to pay annual user fees for marketed products, as well as new application fees for supplemental applications with clinical data.
The FDA strictly regulates the information presented on products on the market, including information on labeling, advertising, and promotion of products. Products may only be promoted for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the rules prohibiting the promotion of off-label uses. A company that improperly promotes off-label uses may be subject to significant liability. Manufacturers must also continue to comply with extensive CGMP regulations, which requires a commitment of time and financial resources. FDA review and approval is generally required for post-approval changes to the manufacturing process and other changes to the approved product, including the addition of new indications and additional labeling claims.
Manufacturers and others involved in the manufacturing and distribution of approved products must register their establishments with the FDA and certain state agencies. The FDA and state agencies may periodically inspect these establishments, sometimes without prior notice, to ensure compliance with CGMP regulations and other obligations. CGMP requirements apply to all stages of the product manufacturing process, including processing, production, sterilization, packaging, labeling, storage and shipment.
Prior FDA approval is often required for changes to the manufacturing process are implemented. FDA regulations require investigation and correction of departures from CGMP requirements. The FDA may also impose reporting and documentation obligations upon the sponsor and any third party manufacturers used by the sponsor. As a result, to remain compliant with CGMP regulations, manufacturers must continue to commit time, effort and financial resources to production and quality control.
The FDA may impose other post-approval requirements as a condition to approving an application, such as post-marketing testing (including Phase 4 clinical trials) and surveillance to monitor and assess the product’s safety and effectiveness upon commercialization.
The FDA may withdraw approval of a product if an applicant fails to maintain compliance with regulatory requirements or if certain issues arise after the product is introduced to the market. For instance, a subsequent discovery of previously unknown issues, including adverse events of unexpected frequency or severity, problems with the manufacturing process, or failure to comply with regulatory requirements, could result in restrictions on the product or a complete withdrawal from the market.
In such cases, potential consequences include revisions to the approved labeling to include new safety information; post-market studies or clinical trials to evaluate new safety risks; and imposition of restrictions under a REMS program. Other potential consequences include:
| ● | Restrictions on the manufacturing or marketing of the product (including complete withdrawal or recall of the product); | |
| ● | Warning letters or holds on post-approval clinical trials; | |
| ● | FDA’s refusal to approve pending NDAs or supplements to approved NDAs; | |
| ● | Suspension or revocation of product license approvals; | |
| ● | Product seizures or detentions; | |
| ● | FDA’s refusal to allow imports or exports of products; or | |
| ● | Civil penalties, criminal penalties or injunctions. |
| 77 |
Manufacturers and distributors must also comply with the Prescription Drug Marketing Act (“PDMA”) and state laws that regulate distribution of prescription products. The PDMA regulates the distribution of prescription drugs, products and product samples at the federal level and sets minimum standards for the registration and regulation of distributors by the states. The PDMA and state laws restrict the distribution of prescription product samples and impose requirements to ensure accountability in distribution.
In addition, new federal legislation and guidance could substantially alter the statutory provisions governing approval, manufacturing and marketing of products regulated by the FDA. New legislation, FDA regulations, guidance, and policies are periodically revised or reinterpreted in ways that could significantly impact our business and our products. We cannot predict the enactment, implementation and potential consequences of any future legislative, regulatory or policy changes.
Pharmaceutical Coverage, Pricing and Reimbursement
In the United States, commercial sales of any products subject to regulatory approval could be conditioned on whether third-party payors (such as government authorities, managed care providers, private health insurers and other organizations) are able to provide coverage and reimbursement in connection with the products.
Coverage and reimbursement of costs are areas of significant uncertainty for any products subject to regulatory approval. The process for determining coverage versus reimbursement may vary widely among third-party payors. Third-party payors may also impose additional requirements on and restrictions to coverage and reimbursement, which could influence the purchase of certain healthcare services and products.
Third-party payors may limit coverage to specific drugs on an approved list, or formulary, which could omit some FDA-approved drugs for a particular indication. Third-party payors may also place drugs at certain formulary levels that result in a lower reimbursement and higher cost-sharing obligation for patients. A third-party payor’s decision to provide coverage for a product may not necessarily imply approval of an adequate reimbursement rate. In addition, the unavailability of third-party reimbursement may affect our ability to maintain price levels sufficient to realize an appropriate return on our investment in product development. Coverage by one third-party payor may not necessarily indicate or imply coverage or reimbursement by other third-party payors. Also, the level or scope of coverage and reimbursement may vary significantly among third-party payors. In addition to scrutinizing the safety and efficacy of medical products and services, third-party payors have increasingly begun to examine and challenge the price, cost-effectiveness and necessity of certain products and services. Thus, to obtain and maintain coverage and reimbursement for any products approved for sale, the conducting of expensive pharmacoeconomic studies may be required to demonstrate the medical necessity and cost-effectiveness of such products. There is a chance that third-party payors may not consider our product medically necessary or cost-effective. If third-party payors make such a determination, they may not cover the product after approval as a benefit under their plans. If third-party payors do cover the product, the returns from sales of our product may not sufficiently yield a profit.
Furthermore, federal and state governmental authorities have increasingly shown an interest in implementing cost containment programs to limit government-paid healthcare costs. Such cost containment programs include restrictions on coverage and reimbursement, price controls and requirements to substitute branded prescription drugs with generic products. The adoption and expansion of such restrictive policies and controls could impose limitations or exclusions from coverage for our product.
In the United States, we expect third-party payors and government authorities to increase emphasis on managed care and cost containment measures, which will impact the pricing and coverage for pharmaceutical products. Coverage policies and third-party reimbursement rates may change at any time. Even if we achieve favorable coverage and reimbursement status for an approved product, less favorable coverage policies and reimbursement rates could still be implemented in the future.
| 78 |
Protection of Intellectual Property
We strive to protect our intellectual property in a variety of ways to promote the development of our product candidate and business. Our strategy to safeguard this intellectual property includes the following:
● Patents and patent applications. We are in the process of obtaining method of use patents intended to cover our ANEB-001 product candidate, which are important to the development of our business. We have filed two patent applications for various methods of use of the ANEB-001 compound and delivery systems, which applications are currently pending before the U.S. Patent and Trademark Office. We intend to pursue foreign jurisdictions for these patent applications at the relevant time. The patents are expected to expire in 2040.
● Regulatory exclusivity. We could obtain regulatory exclusivity in the United States upon receiving approval of our New Drug Application (“NDA”) from the FDA. Upon approval of a new chemical entity (“NCE”), which is a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which the FDA may not approve a generic version of the drug. In addition, in seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application (“ANDA”) and then later challenged pursuant to a paragraph IV certification. As part of the Paragraph IV certification process, an NDA holder may initiate a patent infringement lawsuit against the ANDA applicant. The filing of a patent infringement lawsuit by an NDA holder automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the Orange Book-listed patent, settlement of the lawsuit, or a decision in the infringement case that is favourable to the ANDA applicant. Finally, we could receive an orphan drug designation, which would grant a total of seven years of marketing exclusivity in the United States under the US Orphan Drug Act of 1983, or pediatric drug designation, which provides NDA holders (under the Best Pharmaceuticals for Children Act (BPCA)) a six-month extension of any exclusivity (patent or non-patent) for a drug.
● Trade secrets. We rely on trade secret laws of general applicability for aspects of our business that are not readily amenable to or appropriate for patent protection.
● Confidentiality agreements. We rely upon confidentiality agreements signed by our employees, consultants and third parties.
● License agreement. We have entered into an exclusive worldwide licensing agreement with Vernalis to develop, strengthen and commercialize our ANEB-001 compound. This exclusive in-licensing opportunity allows us to maintain and enhance our proprietary position in ANEB-001.
● Trademarks. We use “Anebulo” as our trademark. As we develop our drug candidate and business, we intend to add trademarks to our portfolio of intellectual property.
We believe these methods provide us material defensibility around our core intellectual property.
Employees
As of March 1, 2021, we had two full-time employees, none of which were covered by collective bargaining agreements. In addition, we have a number of outside consultants that are not on our payroll who are involved directly in scientific research and development activities. We believe that relations with our employees are generally good.
Facilities
We manage our business operations from our principal executive office in Lakeway, Texas, in 700 square feet of leased space under a sublease with JFL Capital Management LLC, a company controlled by Joseph F. Lawler, M.D., Ph.D., the founder and a director of our company. Our office lease extends through August 2021, under which we currently pay approximately $1,200 per month. We believe our present office space is adequate for our current operations and for near-term planned expansion.
Legal Proceedings
There are no legal proceedings or arbitration proceedings currently pending against our company.
| 79 |
Executive Officers and Directors
The following table sets forth certain information regarding our executive officers and directors as of the date of this prospectus:
| Name | Age | Position(s) | ||
| Joseph F. Lawler, M.D., Ph.D. | 48 | Founder and Director | ||
| Daniel Schneeberger, M.D. | 36 | Chief Executive Officer and Director | ||
| Rex Merchant | 61 | Chief Financial Officer | ||
| Jason M. Aryeh | 52 | Director | ||
| Aron R. English | 38 | Director | ||
| Kenneth Lin, M.D. | 47 | Director | ||
| Karah Parschauer | 42 | Director |
We intend to identify and appoint one additional independent director with relevant business experience before the completion of this offering.
The following information provides a brief description of the business experience of each executive officer and director.
Joseph F. Lawler, M.D., Ph.D. founded our company in April 2020 and has been a member of our board of directors since inception. He briefly served as our President from April to June 2020. Dr. Lawler is also the founder and has served as Managing Member of JFL Capital Management LLC, a healthcare investment fund with an emphasis on companies pursuing clinical drug development, since January 2015. Prior to his involvement with JFL Capital Management LLC, Dr. Lawler was a co-founder and served as Senior Managing Partner of Merus Capital Partners, LLC, a proprietary trading business, from October 2011 to November 2014. Dr. Lawler served as portfolio manager at MKM Longboat Capital Advisors LLC, a London-based hedge fund manager, from February 2008 to November 2008. Prior to that, Dr. Lawler was responsible for public and private biotechnology investments as an analyst at Och-Ziff Capital Management Group LLC, a hedge fund manager and global alternative asset management firm, from May 2006 to February 2008. Dr. Lawler served as an analyst at Sagamore Hill Capital Management LP, a multi-strategy hedge fund manager, from March 2005 to May 2006, and also previously served as an associate in the venture capital group at J.P. Morgan Partners, LLC, from March 2003 to March 2005. Dr. Lawler received his M.D. and Ph.D. from The Johns Hopkins University School of Medicine and he earned his B.A. degree from Queens College, City University of New York.
We believe Dr. Lawler’s exceptional credentials and expertise in the biomedical field, coupled with his experience in investment and strategic development, make him well-qualified to serve on the board of directors.
Daniel Schneeberger, M.D. joined our company as Chief Executive Officer and a member of our board of directors in July 2020. Dr. Schneeberger previously spent more than four years as an institutional investor in the biotechnology and healthcare sector, serving as Chief Executive Officer of ADAR1 Capital Management from January 2019 to June 2020, and senior analyst at JFL Capital Management LLC from May 2016 to December 2018, where he specialized in the prediction of clinical drug trial readouts as a basis for investments. Prior to his involvement with JFL Capital Management LLC, Dr. Schneeberger was a consultant at McKinsey & Company from April 2013 to May 2016. There, he advised clients in the pharmaceutical, private equity and agrochemical industry on research and development, portfolio decisions and commercial strategies. Dr. Schneeberger received his medical diploma and a doctorate in rheumatology from the University of Basel, Switzerland. He also earned an M.B.A. from Harvard Business School and was named a Baker Scholar.
We believe Dr. Schneeberger’s experience in private equity investing and operational and financial consulting in the biotechnology industry (with a focus on the commercialization of drugs) makes him well-qualified to serve on the board of directors.
| 80 |
Rex Merchant joined our company as Chief Financial Officer in January 2021. He has served as the Chief Financial Officer of JFL Capital Management LLC since May 2018 and of various other investment advisors and non-profit organizations since 1998. Prior to joining JFLCapital Management, Mr. Merchant served as Chief Financial Officer of Western Investment LLC, a hedge fund manager and investment advisory firm, from September 2008 to December 2017. Mr. Merchant also served as Chief Financial Officer of Leadership Foundations, a non-profit organization, from October 2011 to April 2018. He also has performed business valuation, litigation analysis and expert witness services, as well as extensive work in information technology throughout his career. Mr. Merchant received an M.S. degree in Taxation from Golden Gate University, a B.S. degree in Industrial Engineering from Stanford University, and he holds Chartered Financial Analyst (CFA) and Chartered Alternative Investment Analyst (CAIA) designations.
Jason M. Aryeh joined our board of directors in March 2021. He is the founder and managing general partner of JALAA Equities, LP, a private hedge fund focused on the biotechnology and medical device sectors, and has served in such capacity since 1997. Mr. Aryeh has served as a member of the board of directors of Ligand Pharmaceuticals Inc., a publicly-traded biopharmaceutical company focused on developing or acquiring technologies that help pharmaceutical companies discover and develop medicines, since September 2006. Mr. Aryeh has also served as a director of Orchestra BioMed, Inc., a private biomedical innovation company focused on developing transformative therapeutic products, since November 2018. Mr. Aryeh has served as a director of numerous public and private companies. Mr. Aryeh earned a B.A. in economics, with honors, from Colgate University, and is a member of the Omnicron Delta Epsilon Honor Society in economics.
We believe that Mr. Aryeh’s in-depth knowledge of the biopharmaceutical market and broad range of companies in the industry as the managing general partner of a hedge fund focused on the life sciences sector make him well qualified as a member of our board. He also brings transactional expertise in capital markets.
Aron R. English has been a member of our board of directors since June 2020. He is the founder and has served as the President and Portfolio Manager of 22NW, LP, a Seattle-based value fund specializing in small and microcap investments with a multi-year investment horizon, since August 2014. Previously, Mr. English served as the director of research at Meson Capital Partners LLC, an investment firm, from January 2014 to August 2014. Prior to that, he served as director of research at RBF Capital, LLC, a provider of wealth management and financial services, from September 2010 until December 2013, after initially serving as a research analyst at the firm from September 2008 to September 2010. Mr. English served as a research assistant at McAdams Wright Ragen Inc., an investment firm, from March 2006 until September 2008. Mr. English earned his B.A. degree in English Literature with honors from the University of Washington.
We believe that Mr. English’s investment experience and knowledge of the capital markets will make him a valuable addition to the board of directors.
Kenneth Lin, M.D. joined our board of directors in February 2021. From January 2015 to July 2019, Dr. Lin founded and served as the President and CEO of Ab Initio Biotherapeutics, a biologics discovery company targeting G protein coupled receptors, through to its sale to Ligand Pharmaceuticals. From July 2012 to July 2014, Dr. Lin was the Vice President of Corporate Development and Investor Relations for Ulthera, Inc., a medical device company that was acquired by Merz Pharma. From April 2008 to June 2012, Dr. Lin was a Vice President at TPG, a private equity investment firm, where he focused on healthcare. From August 2003 to June 2007, Dr. Lin was an associate at JPMorgan Partners, a private equity investment firm. From September 2000 to June 2003, he was an associate in the Global Equity Research Division of Goldman Sachs. Dr. Lin received his M.D. from Case Western Reserve University with honors and his B.S. degree in Biological Sciences from Stanford University.
We believe Dr. Lin’s extensive experience with private equity investing and management of biotechnology companies makes him well-qualified to serve on the board of directors.
Karah Parschauer joined our board of directors in February 2021. Since June 2016, Ms. Parschauer has served as General Counsel and Executive Vice President of Ultragenyx Pharmaceutical, Inc., a clinical-stage biopharmaceutical company. Prior to Ultragenyx, Ms. Parschauer served in various executive capacities, and most recently as Vice President, Associate General Counsel, at Allergan plc, a pharmaceutical company, from June 2005 until June 2016. Prior to Allergan, Ms. Parschauer was an attorney at Latham & Watkins LLP, where she practiced in the areas of mergers and acquisitions, securities offerings, and corporate governance. She has served as a member of the board of directors of Evolus, Inc., a medical aesthetics company, since July 2019 and of Arcturus Therapeutics, Ltd., a clinical-stage messenger RNA medicines company, since June 2019. Ms. Parschauer holds a B.A. degree in Biology from Miami University and a J.D. from Harvard Law School.
We believe Ms. Parschauer’s extensive experience within the pharmaceutical industry and as an attorney, particularly with respect to matters concerning corporate governance, makes her a valuable addition to the board of directors.
Board Composition
Our business and affairs are managed under the direction of our board of directors, which currently consists of six members. The number of directors is determined by our board of directors, subject to the terms of our amended and restated certificate of incorporation and bylaws that will become effective upon the completion of this offering. Upon the completion of this offering, our board of directors will consist of seven members.
| 81 |
Our board of directors will be divided into three classes as nearly equal in size as is practicable. The composition of the board of directors immediately following the offering will be as follows:
● Class I, which will initially consist of and , whose terms will expire at our annual meeting of stockholders to be held in 2022;
● Class II, which will initially consist of and , whose terms will expire at our annual meeting of stockholders to be held in 2023; and
● Class III, which will initially consist of , and whose terms will expire at our annual meeting of stockholders to be held in 2024.
Upon the expiration of the initial term of office for each class of directors, each director in such class shall be elected for a term of three years and serve until a successor is duly elected and qualified or until his or her earlier death, resignation or removal. Vacancies occurring on the board of directors, whether due to death, resignation, removal, retirement, disqualification or for any other reason, and newly created directorships resulting from an increase in the authorized number of directors, may be filled by a majority of the remaining members of the board of directors. Directors may be removed, but only for cause, with the affirmative vote of the holders of a majority of the voting power of our common stock.
Director Independence
Upon the completion of this offering, our common stock will be listed on The Nasdaq Capital Market (“Nasdaq”). Under Nasdaq rules, independent directors must comprise a majority of a listed company’s board of directors within a specified period after completion of this offering. In addition, Nasdaq rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and governance committees must be independent. Under Nasdaq rules, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Audit committee members of a listed company must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (i) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, our board of directors has determined that Karah Parschauer, Kenneth Lin, M.D., Jason M. Aryeh and , representing a majority of our directors, do not have any relationships that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under Nasdaq rules and Rule 10A-3 of the Exchange Act. In making these determinations, our board of directors considered the relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Board Committees
Upon the closing of this offering, our board of directors will have three standing committees: an audit committee, a compensation committee and a nominating and corporate governance committee. Under Nasdaq rules and Rule 10A-3 of the Exchange Act, the membership of the audit committee is required to consist entirely of independent directors, subject to applicable phase-in periods. The following is a brief description of our committees.
Audit committee. In accordance with our audit committee charter, after this offering, our audit committee will: oversee our corporate accounting and financial reporting processes and our internal controls over financial reporting; evaluate our independent public accounting firm’s qualifications, independence and performance; engage and provide for the compensation of our independent public accounting firm; approve the retention of our independent public accounting firm to perform any proposed permissible non-audit services; review our financial statements; review our critical accounting policies and estimates and internal controls over financial reporting; and discuss with management and our independent registered public accounting firm the results of the annual audit and the reviews of our quarterly financial statements. We believe that our audit committee members meet the requirements for financial literacy under the current requirements of the Sarbanes-Oxley Act, Nasdaq and SEC rules and regulations. In addition, the board of directors has determined that is qualified as an audit committee financial expert within the meaning of SEC regulations. We have made this determination based on information received by our board of directors. The audit committee will be composed of (Chair), Dr. Lin and .
| 82 |
Compensation committee. In accordance with our compensation committee charter, after this offering, our compensation committee will review and recommend policies relating to compensation and benefits of our officers and employees, including reviewing and approving corporate goals and objectives relevant to compensation of the Chief Executive Officer and other senior officers, evaluating the performance of these officers in light of those goals and objectives and setting compensation of these officers based on such evaluations. The compensation committee will also administer the issuance of stock options and other awards under our equity-based incentive plans. We believe that the composition of our compensation committee meets the requirements for independence under, and the functioning of our compensation committee complies with, any applicable requirements of the Sarbanes-Oxley Act, Nasdaq and SEC rules and regulations. We intend to comply with future requirements to the extent they become applicable to us. The compensation committee will be composed of (Chair) and Dr. Lin.
Nominating and governance committee. In accordance with our nominating and governance committee charter, after this offering, our nominating and governance committee will recommend to the board of directors nominees for election as directors, and meet as necessary to review director candidates and nominees for election as directors; recommend members for each committee of the board of directors; oversee corporate governance standards and compliance with applicable listing and regulatory requirements; develop and recommend to the board of directors governance principles applicable to the company; and oversee the evaluation of the board of directors and its committees. We believe that the composition of our nominating and governance committee meets the requirements for independence under, and the functioning of our nominating and governance committee complies with, any applicable requirements of the Sarbanes-Oxley Act, Nasdaq and SEC rules and regulations. We intend to comply with future requirements to the extent they become applicable to us. The nominating and governance committee will be composed of Ms. Parschauer (Chair) and .
Code of Business Conduct and Ethics
We will adopt a new code of business conduct and ethics that applies to all of our officers, directors and employees, including our principal executive officer, principal financial officer, principal accounting officer and controller, or persons performing similar functions, which will be posted on our website. Our code of business conduct and ethics is a “code of ethics,” as defined in Item 406(b) of Regulation S-K. The information contained on, or accessible from, our website is not part of this prospectus by reference or otherwise. We will make any legally required disclosures regarding amendments to, or waivers of, provisions of our code of business conduct and ethics on our website.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee is an executive officer or employee of our company. None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Limitations on Director and Officer Liability and Indemnification
Our certificate of incorporation limits the liability of our directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except liability for:
| ● | any breach of their duty of loyalty to the corporation or its stockholders; | |
| ● | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; | |
| ● | unlawful payments of dividends or unlawful stock repurchases or redemptions; or | |
| ● | any transaction from which the director derived an improper personal benefit. |
| 83 |
Our certificate of incorporation and our bylaws provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Any repeal of or modification to our certificate of incorporation and our bylaws may not adversely affect any right or protection of a director or officer for or with respect to any acts or omissions of such director or officer occurring prior to such amendment or repeal. Our bylaws also provide that we shall advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in connection with their services to us, regardless of whether our bylaws permit such indemnification.
Prior to the completion of this offering, we intend to enter into separate indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our bylaws. These agreements, among other things, provide that we will indemnify our directors and executive officers for certain expenses (including attorneys’ fees), judgments, fines, penalties and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of such person’s services as one of our directors or executive officers, or any other company or enterprise to which the person provides services at our request. We believe that these provisions and agreements are necessary to attract and retain qualified persons as directors and executive officers.
The limitation of liability and indemnification provisions that will be contained in our certificate of incorporation and our bylaws upon completion of this offering may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. There is no pending litigation or proceeding involving one of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
| 84 |
Summary Compensation Table
The following table sets forth summary compensation information for the following persons: (i) all persons serving as our principal executive officer during the period from April 23, 2020 (date of inception) to June 30, 2020, and (ii) our two other most highly compensated executive officers who received compensation during the period from April 23, 2020 (date of inception) to June 30, 2020 of at least $100,000 and who were executive officers on June 30, 2020. We refer to these persons as our “named executive officers” in this prospectus. The following table includes all compensation earned by the named executive officers for the respective period, regardless of whether such amounts were actually paid during the period:
| Name and Position | Fiscal Years | Salary($) | Bonus($) | Stock Awards($) | Option Awards($) | All Other Compensation($) | Total($) | |||||||||||||||||||||
| Daniel Schneeberger, M.D. | 2020 | - | - | - | - | - | - | |||||||||||||||||||||
| Chief Executive Officer | ||||||||||||||||||||||||||||
| Joseph F. Lawler, M.D., Ph.D. | 2020 | - | - | - | - | - | - | |||||||||||||||||||||
| Former President |
Employment Agreements
On July 21, 2020, we entered into an employment agreement with Daniel Schneeberger, M.D. Pursuant to the employment agreement, Dr. Schneeberger agreed to serve as our Chief Executive Officer, oversee our day-to-day business operations, including directing research and development of our medical technologies, and perform duties customary for this position. The employment agreement with Dr. Schneeberger is effective for an initial term of three years, commencing on July 21, 2020 and concluding on August 1, 2023, with automatic extensions for successive one-year periods, unless earlier terminated. The employment agreement provides Dr. Schneeberger with a base salary of $7.25 per hour paid in accordance with our customary payroll practices, as well as stock-based awards subject to our 2020 Stock Incentive Plan (the “Incentive Plan”). Subject to the Incentive Plan, Dr. Schneeberger will be entitled to a total of 40,937 shares of our common stock, which will vest ratably in six quarterly installments (approximately 6,822 shares each quarter until the final quarter award of 6,827 shares) over an 18-month period. In addition, Dr. Schneeberger will be entitled to stock-based awards based on achieving certain performance targets as follows: (i) 40,937 shares upon the first patient being dosed in a Phase 2 clinical trial with ANEB-001; (ii) 40,937 shares upon the availability of a newly synthesized active pharmaceutical ingredient acceptable for dosing in a U.S. clinical trial; and (iii) 40,939 shares upon the completion of an initial public offering and a public listing on a major exchange.
In the event of a change in control of our company, Dr. Schneeberger will be entitled to the vesting of 50% of any stock-based awards granted but not yet vested prior to the change in control event not less than six months after the change in control event, provided Dr. Schneeberger remains employed by our company. If the change in control event is an initial public offering, Dr. Schneeberger will be entitled to the full vesting of any stock-based awards. In the event of Dr. Schneeberger’s termination, Dr. Schneeberger will be entitled to severance payments as follows: (i) if terminated by us without cause or upon his resignation for good reason, severance payments will be equal to the remainder of the annual base compensation for the year in which the date of termination occurs and the immediate award and vesting of the next quarterly stock-based award; and (ii) if terminated due to non-extension of the initial term, and only if we exercise our non-compete option, severance payments will be equal to the annual base compensation for the year in which the date of termination occurs, multiplied by a fraction, the numerator of which is equal to the number of days from the date of termination through the one-year anniversary thereof and the denominator of which is 365.
The employment agreement with Dr. Schneeberger also contains covenants (a) restricting Dr. Schneeberger from engaging in any activities competitive with our business during his employment with us and for a period of one year thereafter, (b) preventing Dr. Schneeberger from recruiting, soliciting or hiring away employees of our company for a period of one year after his employment with us, (c) prohibiting Dr. Schneeberger from disclosing confidential information regarding our company at any time, and (d) confirming that all work product or other intellectual property developed by Dr. Schneeberger and relating to our business constitutes our sole and exclusive property. The employment agreement is governed by the laws of the state of Texas.
| 85 |
Outstanding Equity Awards at June 30, 2020
The following table shows outstanding option awards held by the named executive officers as of June 30, 2020.
| Name | Vested Shares | Unvested Shares | Total Shares | |||||||||
| Daniel Schneeberger, M.D. | — | — | — | |||||||||
| Joseph F. Lawler, M.D., Ph.D. | — | — | — |
2020 Stock Incentive Plan
On June 17, 2020, our board of directors and stockholders adopted our 2020 Stock Incentive Plan (the “Plan”). The purpose of the Plan is to enhance our ability to attract, retain and motivate persons who are expected to make important contributions to our company and by providing such persons with equity ownership opportunities and performance-based incentives that are intended to better align the interests of such persons with those of our stockholders. We have reserved a total of 275,000 shares of common stock for issuance under the Plan.
No stock-based awards were issued under the Plan for the period from April 23, 2020 (date of inception) to June 30, 2020. Subsequent to June 30, 2020, we awarded 163,750 shares of restricted common stock to Dr. Schneeberger, subject to the satisfaction of certain performance targets and vesting requirements pursuant to the award agreement and our employment agreement with Dr. Schneeberger.
Administration. The Plan is to be administered by the board of directors. Subject to the terms of the Plan, the board of directors is authorized to grant awards; adopt, amend and repeal such administrative rules, guidelines and practices relating to the Plan as it deems advisable; construe and interpret terms of the Plan and any award agreements entered into under the Plan; correct any defect; supply any omission; reconcile any inconsistency in the Plan or any award in the manner and to the extent it deems expedient. All decisions by the board of directors shall be final and binding on all persons having a claim or interest in the Plan or in any award. To the extent permitted by applicable law, the board of directors is authorized to delegate any or all of its powers under the Plan to one or more committees or subcommittees of the board of directors.
Eligibility. The persons eligible to receive awards under the Plan are our employees, officers, directors, consultants and advisors.
Types of Awards. Our Plan provides for the issuance of common stock, stock options, stock incentive options, restricted stock, restricted stock units, and other stock-based awards.
Stock Available for Awards. Subject to certain adjustments, the total number of shares of common stock that may be awarded under the Plan will be equal to 275,000 shares. In the event an award expires, lapses, is forfeited, or is terminated, surrendered, or canceled without having been fully exercised, the unused common stock covered by such award shall again be available to be granted under the Plan. Shares of common stock delivered or tendered to satisfy any applicable tax withholding obligation shall be added to the number of shares of common stock available to be granted under the Plan, except in the case of Incentive Stock Options, which are subject to the limitations in the Internal Revenue Code of 1986 (the “Code”). Shares of common stock issued under the Plan may consist in whole or in part of authorized but unissued shares, shares purchased on the open market, or treasury shares. Any participant under the Plan who was a resident of the State of California on the date of the grant of an option shall be subject to the conditions and exclusions of Section 260.140.45 of the California Code of Regulations (the “California Regulations”), based on our shares which are outstanding at the time the calculation is made. After taking into consideration the 163,750 shares of restricted common stock awarded to Dr. Schneeberger, the Plan has 111,250 shares remaining available.
In the event of a merger or consolidation of an entity with us or the acquisition by us of property or stock of an entity, the board of directors may grant awards in substitution for any options or other stock or stock-based awards granted prior to such merger or consolidation. Such substitute awards may be granted on such terms as the board of directors deems appropriate in the circumstances, notwithstanding any limitations on Awards contained in the Plan.
Stock Options. The board of directors is authorized to grant options to purchase common stock and determine the number of shares of common stock to be covered by each option, the exercise price of each option, and the conditions and limitations applicable to the exercise of each option, including conditions relating to applicable federal or state securities laws, as the board of directors considers necessary or advisable.
| 86 |
Incentive stock options, as defined in Section 422 of the Code, are only available to our employees. All such incentive stock options shall be subject to and shall be construed consistently with the requirements of Section 422 of the Code. If an option intended to qualify as an incentive stock option does not so qualify, the board of directors has discretion to amend the Plan and award with respect to such option so that such option qualifies as an incentive stock option.
The board of directors is authorized to establish the exercise price of each option and specify the exercise price in an applicable option agreement. The exercise price is not to be less than 100% of the fair market value on the date the option is granted, but in the case of an incentive stock option granted to an employee who owns stock representing more than 10% of the voting power of all classes of our stock, the per share exercise price is to be no less than 110% of the fair market value on the date the option is granted. The board of directors may specify the terms and duration under which options are exercisable in an applicable option agreement, but the maximum term is 10 years for the exercise of options and 5 years for the exercise of incentive stock options.
Restricted Stock; Restricted Stock Units. The board of directors is authorized to grant restricted stock and restricted stock units and to determine the terms and conditions set forth in the applicable award agreement, including the conditions for vesting, repurchase, forfeiture and issue price. Restricted stock is a grant of shares of common stock which are subject to our right to repurchase at their issue price or other stated formula, and which may be forfeited if issued at no cost, under conditions specified by the board of directors. Alternatively, the board of directors may grant restricted stock units, which entitle the recipient to receive common stock or cash at the time such award vests. Participants holding restricted stock or restricted stock units are entitled to ordinary cash dividends. Prior to settlement, an award of restricted stock units carries no voting or dividend rights or other rights associated with share ownership, although dividend equivalents may be granted.
Other Stock-Based Awards. The board of directors is authorized to grant awards that are valued by reference to, or otherwise based on, shares of common stock, including stock appreciation rights and awards entitling recipients to receive shares of common stock to be delivered in the future. The board of directors has the sole discretion to determine the terms and conditions of such awards, including purchase price, transfer restrictions, and vesting conditions.
Adjustments for Changes in Common Stock and Certain Other Events. In the event of any stock split, reverse stock split, stock dividend, recapitalization, combination of shares, reclassification of shares, spin-off or other similar change in capitalization or event, or any dividend or distribution to holders of common stock other than an ordinary cash dividend, we will equitably adjust in the manner determined by the board of directors (i) the number and class of securities available under the Plan, (ii) the number and class of securities and exercise price per share of each outstanding option, (iii) the number of shares subject to and the repurchase price per share subject to each outstanding restricted stock award, and (iv) the terms of each other outstanding award.
General Provisions Applicable to Awards. Awards are subject to restrictions not to be sold, assigned, transferred, pledged or otherwise encumbered, unless the board of directors determines otherwise. The board of directors is authorized to determine the form in which each award shall be evidenced (written, electronic or otherwise), the terms of each award, and the effect of an award in the event a recipient’s disability, death, retirement, termination, cessation of employment, authorized leave of absence, other change in employment or other change in status. The board of directors may provide that any award shall become immediately exercisable in full or in part, free from some or all restrictions, or otherwise realizable at any time.
No Rights as Stockholder. Subject to the provisions of the applicable award, recipients of an award under the Plan (including their designated beneficiaries) have no rights as stockholders with respect to such award until becoming the record holder of shares of common stock to be distributed with respect to such award.
Effective Date and Term of the Plan. The Plan is effective on the date adopted by the board of directors and expires in ten years.
Amendment of the Plan. The board of directors may amend, suspend or terminate the Plan (or any portion thereof) at any time, subject to the approval of stockholders or provisions of the Code, as applicable.
Compliance with Code Section 409A. Unless otherwise provided for in an award, awards granted under the Plan are intended to be exempt from Section 409A of the Code, and, to the extent not so exempt, in compliance with Section 409A of the Code.
Restrictions on Shares; Claw-back Provisions. The Plan and awards granted thereunder are subject to such terms and conditions determined by the board of directors, including restrictions on the transferability of shares, our right to repurchase shares, our right to require the transfer of shares in the event of certain transactions, tag-along rights, bring-along rights, redemption and co-sale rights and voting requirements. The issuance of shares of common stock are subject to recipients’ consent to such terms and conditions. All awards are subject to the provisions of any claw-back policy implemented by us, including any claw-back policy adopted to comply with the requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act and any rules or regulations promulgated thereunder, to the extent set forth in such claw-back policy and/or in the applicable award agreement.
Director Compensation
As of December 31, 2020, no fees, equity awards or other compensation was paid to our directors for their services as directors.
In 2021, we have begun, following the commencement of a non-employee director’s service as a director, to grant the director an option to purchase such number of shares of our common stock as has a grant date fair market value of approximately $79,000, applying a customary Black-Scholes calculation, at an exercise price per share of the option not less than the fair market value of our common stock on the grant date. The option will be subject to the terms and conditions applicable to options granted under our 2020 Stock Incentive Plan (as amended from time to time) as described in the Plan and the applicable stock option agreement. The shares underlying the option will vest 25% on the one-year anniversary of the grant date and on a straight-line monthly basis over the next four years of continuous service, as described in the applicable stock option agreement. Additionally, the option will be subject to full acceleration upon the consummation of a reorganization event (as defined in the Plan). Subject to continued service as a director and the approval of our board or our compensation committee, it is anticipated that non-employee directors will be eligible on an annual basis for additional grants of a similar aggregate value.
Further, we intend to compensate each non-employee director $1,000 per year in cash payable on a quarterly basis in advance for the director’s service as a member of the board and, as applicable, an additional $10,000 per year, payable on a quarterly basis in advance for the director’s service as the Chairman of the Board or chairman of any committee of the board.
| 87 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Policies and Procedures for Transactions with Related Persons
Our board of directors intends to adopt a written related person transaction policy to set forth the policies and procedures for the review and approval or ratification of related person transactions. Related persons include any executive officer, director or a holder of more than 5% of our common stock, including any of their immediate family members and any entity owned or controlled by such persons. A related person transaction refers to any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which (i) we were or are to be a participant, (ii) the amount involved exceeds $120,000, and (iii) a related person had or will have a direct or indirect material interest. Related person transactions include, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness, and employment by us of a related person, in each case subject to certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act.
We expect that the policy will provide that in any related person transaction, our audit committee and board of directors will consider all of the available material facts and circumstances of the transaction, including: the direct and indirect interests of the related persons; in the event the related person is a director (or immediate family member of a director or an entity with which a director is affiliated), the impact that the transaction will have on a director’s independence; the risks, costs and benefits of the transaction to us; and whether any alternative transactions or sources for comparable services or products are available. After considering all such facts and circumstances, our audit committee and board of directors will determine whether approval or ratification of the related person transaction is in our best interests. For example, if our audit committee determines that the proposed terms of a related person transaction are reasonable and at least as favorable as could have been obtained from unrelated third parties, it will recommend to our board of directors that such transaction be approved or ratified. In addition, once we become a public company, if a related person transaction will compromise the independence of one of our directors, our audit committee may recommend that our board of directors reject the transaction if it could affect our ability to comply with securities laws and regulations or Nasdaq listing requirements.
Each transaction described in “Certain Relationships and Related Transactions” was entered into prior to the adoption of our audit committee charter and the foregoing policy proposal.
Transactions and Relationships with Directors, Officers and 5% Stockholders
On May 28, 2020 and June 18, 2020, we executed two promissory notes payable to Dr. Lawler in the aggregate principal amount of $200,000, reflecting cash advances by the lender to us in May and June 2020. The indebtedness is unsecured and bears interest at the rate of 8.0% per year. All accrued and unpaid interest and principal on the promissory note issued on May 28, 2020 is due and payable on demand by the holder on or after the date on which we consummate an equity financing (or series of equity financings having materially similar terms and conditions) pursuant to which we sell and issue shares of preferred stock for total aggregate gross proceeds of at least $2,500,000. As of the date of this prospectus, the related party investor has not yet demanded repayment of the note.
All accrued and unpaid interest and principal on the promissory note issued on June 18, 2020 is due and payable on demand by the holder on June 17, 2023. All accrued and unpaid interest and principal under both promissory notes shall be automatically due upon a change in control, defined generally as a consolidation or merger of our company, any transaction or series of transactions in which in excess of 50% of our voting power is transferred, a sale of all or substantially all of our assets or an exclusive license of all or substantially all our material intellectual property. We have used the proceeds of the promissory notes to fund organizational costs and expenses.
On June 18, 2020, we received gross proceeds of $3,000,000 from a private placement of our series A preferred stock (the “Private Placement”), convertible into 341,250 shares of our common stock, pursuant to the terms of a Securities Purchase Agreement with 22NW, LP, an institutional accredited investor affiliated with Aron R. English, who became a director of our company at such time. The series A preferred stock is convertible into shares of common stock automatically upon the closing of this offering. The conversion price of the series A preferred stock is $8.7912 per share. The conversion price is subject to adjustment if, at any time prior to conversion of the shares, we issue in a financing additional shares of common stock or other equity or equity-linked securities at a purchase, conversion or exercise price less than $8.7912 per share. In any such case, we have agreed to issue additional shares of series A preferred stock to the investors so that the effective purchase price per share in the Private Placement is reduced by a weighted-average anti-dilution percentage that takes into account both the lower per share purchase, conversion or exercise price and the number of such additional shares issued at the lower price. No adjustment will be made, however, in respect of shares of common stock or stock options issued to employees, directors or consultants, or in connection with acquisitions of other corporations or strategic collaborations approved by our board of directors.
| 88 |
As part of the Private Placement, 22NW, LP and Mr. English, individually, further agreed under the Securities Purchase Agreement to purchase upon the achievement of certain corporate events “milestone” warrants for $1.95754 per warrant (or $2,250,000 in the aggregate). The warrants are exercisable for cash for up to 1,149,401 shares of series A preferred stock at an exercise price of $10.11 per share or on a “net-exercise” basis into such lesser number of shares of series A preferred stock by surrendering a portion of the underlying warrant shares, based on the positive difference between the stated warrant exercise price and the initial public offering price per share in this offering, to pay the exercise price. The warrants must be purchased upon our achievement of (i) a filing with the FDA of an investigational new drug application or the making of an analogous regulatory filing in any foreign jurisdiction, whichever is earlier, and (ii) an arrangement by us to produce the active pharmaceutical ingredient of ANEB-001 in amounts sufficient to facilitate the consummation of a trial pursuant to such regulatory filing. The milestone warrants may also be purchased at any time at the option of 22NW, LP and Mr. English.
We lease our office space in Lakeway, Texas from JFL Capital Management LLC, a company controlled by Dr. Lawler, the founder and a director of our company. Our office lease extends through August 2021, under which we currently pay approximately $1,200 per month.
Indemnification Agreements
We have entered or will enter into an indemnification agreement with each of our directors and executive officers. The indemnification agreements and our certificate of incorporation and bylaws require us to indemnify our directors and executive officers to the fullest extent permitted by Delaware law. See “Management — Limitations on Director and Officer Liability and Indemnification.”
| 89 |
The following table and accompanying footnotes set forth certain information with respect to the beneficial ownership of our common stock as of March 12, 2021, referred to below as the “Beneficial Ownership Date,” and as adjusted to reflect the sale of shares of our common stock offered by this prospectus and other transactions occurring before or contemporaneously with this offering, by:
| ● | each person, or group of affiliated persons, who is known to us to be the beneficial owner of 5% or more of the outstanding shares of our common stock; | |
| ● | each of our current directors and each of our named executive officers; and | |
| ● | all our current directors and executive officers as a group. |
Beneficial ownership of shares is determined in accordance with the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to stock options or warrants held by that person that are currently exercisable or exercisable within 60 days after the Beneficial Ownership Date and shares of restricted stock subject to vesting until the occurrence of certain events, including the closing of this offering, are deemed outstanding for computing the beneficial ownership of such person, but are not deemed outstanding for computing the percentage ownership of any other person. Percentage of beneficial ownership is based on shares of common stock outstanding as of the Beneficial Ownership Date and shares of common stock outstanding immediately after this offering, assuming that the underwriters do not exercise their option to purchase up to additional shares of our common stock from this offering in full.
To our knowledge, except as set forth in the footnotes to this table and subject to applicable community property laws, we believe each person named in the table below has sole voting and investment power with respect to the shares set forth opposite such person’s name. Except as otherwise indicated below, the address of each beneficial owner is c/o Anebulo Pharmaceuticals, Inc., 1415 Ranch Road 620 South, Suite 201, Lakeway, TX 78734. The address of 22NW, LP is 1455 NW Leary Way, Suite 400, Seattle, WA 98107.
| Shares of Common Stock Beneficially Owned Immediately Before this Offering | Shares of Common Stock Beneficially Owned Immediately After this Offering | |||||||||||||||
| Name and Address of Beneficial Owner | Number of Shares | Percentage | Number of Shares | Percentage | ||||||||||||
| Directors and Executive Officers: | ||||||||||||||||
| Joseph F. Lawler, M.D., Ph.D. | 2,000,000 | 92.4 | % | |||||||||||||
| Daniel Schneeberger, M.D. (1) | 163,750 | * | ||||||||||||||
| Rex Merchant | - | - | ||||||||||||||
| Jason M. Aryeh | - | - | ||||||||||||||
| Aron R. English (2) | 341,250 | 13.6 | % | |||||||||||||
| Kenneth Lin, M.D. | - | - | ||||||||||||||
| Karah Parschauer | - | - | ||||||||||||||
| 5% Stockholders: | ||||||||||||||||
| 22NW, LP (3) | 341,250 | 13.6 | % | |||||||||||||
| All directors and executive officers as a group ( 7 persons) | 2,505,000 | |||||||||||||||
| * | Represents less than 1% of outstanding shares of common stock. |
| 90 |
| (1) | Consists of 40,937 shares of restricted common stock issued pursuant to the terms of Dr. Schneeberger’s employment agreement with us, which vest in equal quarterly installments from August 1, 2020 to November 1, 2021. In addition, includes additional shares of restricted common stock which vest upon achieving certain performance targets as follows: (i) 40,937 shares upon the first patient being dosed in a Phase 2 clinical trial with ANEB-001, (ii) 40,937 shares upon the availability of a newly synthesized active pharmaceutical ingredient acceptable for dosing in a U.S. clinical trial, and (iii) 40,939 shares issuable upon the completion of an initial public offering or a public listing on a major exchange. |
| (2) | Represents shares of common stock issuable upon automatic conversion of our series A preferred stock upon the closing of this offering owned of record by 22NW, LP, a Delaware limited partnership, of which Mr. English is the President and has voting power and investment power with respect to such shares. Excludes (under “Shares of Common Stock Beneficially Owned Immediately Before this Offering”) milestone warrants to purchase 1,021,690 shares and 127,711 shares of series A preferred stock at an exercise price of $10.11 per share. The warrants may be exercised on a “net-exercise” basis into such lesser number of shares of series A preferred stock by surrendering a portion of the underlying warrant shares, based on the positive difference between the stated warrant exercise price and the initial public offering price per share in this offering, to pay the exercise price. |
| (3) | Assumes the conversion of our series A preferred stock held by 22NW, LP into 341,250 shares of our common stock automatically upon the closing of this offering, but excludes (under “Shares of Common Stock Beneficially Owned Immediately Before this Offering”) milestone warrants to purchase 127,711 shares of series A preferred stock at an exercise price of $10.11 per share. |
| 91 |
The following description summarizes important terms of our capital stock. For a complete description, you should refer to our certificate of incorporation and bylaws, forms of which are incorporated by reference to the exhibits to the registration statement of which this prospectus is a part, as well as the relevant portions of the Delaware law. References to our certificate of incorporation and bylaws are to our certificate of incorporation and our bylaws, respectively, each of which will become effective upon completion of this offering.
General
The following description of our capital stock is a summary and is qualified in its entirety by reference to our amended and restated certificate of incorporation and our bylaws, the forms of which are filed as exhibits to the registration statement of which this prospectus forms a part.
Our authorized capital stock presently consists of 3,800,000 shares of common stock, par value $0.001 per share, and 1,490,651 shares of preferred stock, par value $0.0001 per share, all of which shares of preferred stock have been designated series A preferred stock. Our board of directors may establish the rights and preferences of the preferred stock from time to time. As of January 27, 2021, there were 2,163,750 shares of common stock outstanding, held of record by two stockholders, and 341,250 shares of series A preferred stock outstanding, held of record by one stockholder.
We intend to effect a forward stock split of our outstanding shares of common stock and file with the Delaware Secretary of State an amended and restated certificate of incorporation prior to the completion of this offering.
Upon closing of this offering, our authorized capital stock will consist of shares of common stock, par value $0.001 per share, of which shares will be outstanding, and shares of preferred stock, par value $0.0001 per share, none of which shares will be or outstanding.
Common Stock
Each holder of our common stock is entitled to one vote for each share on all matters to be voted upon by the stockholders and there are no cumulative rights. Subject to any preferential rights of any outstanding preferred stock, holders of our common stock are entitled to receive ratably the dividends, if any, as may be declared from time to time by the board of directors out of legally available funds. If there is a liquidation, dissolution or winding up of our company, holders of our common stock would be entitled to share in our assets remaining after the payment of liabilities and any preferential rights of any outstanding preferred stock.
Holders of our common stock have no preemptive or conversion rights or other subscription rights, and there are no redemption or sinking fund provisions applicable to the common stock. All outstanding shares of our common stock will be fully paid and non-assessable. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock which we may designate and issue in the future.
Preferred Stock
Immediately prior to the date of this prospectus, we were authorized to issue up to 1,490,651 shares of preferred stock, of which 341,250 shares of series A preferred stock were outstanding. Effective upon the closing of this offering, the series A preferred stock will be automatically converted into shares of our common stock and retired, and we will continue to be authorized to issue 1,490,651 shares of “blank check” preferred stock, defined as shares of preferred stock initially authorized by stockholders to be issued by our board of directors with terms and conditions determined by the board but without further action by stockholders. Under the terms of our certificate of incorporation, our board of directors is authorized to issue shares of preferred stock in one or more series without stockholder approval. Our board of directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of preferred stock.
| 92 |
The purpose of authorizing our board of directors to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while providing flexibility in connection with possible future acquisitions and other corporate purposes, will affect, and may adversely affect, the rights of holders of common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of common stock until the board of directors determines the specific rights attached to that preferred stock. The effects of issuing preferred stock could include one or more of the following:
| ● | restricting dividends on the common stock; | |
| ● | diluting the voting power of the common stock; | |
| ● | impairing the liquidation rights of the common stock; or | |
| ● | delaying or preventing changes in control or management of our company. |
We have no present plans to issue any shares of preferred stock.
Milestone Warrants
As part of a 2020 private placement of our series A preferred stock and pursuant to the terms of a securities purchase agreement, dated June 18, 2020, each of 22NW, LP and Aron English, a member of our board, agreed to purchase upon the achievement of certain corporate events “milestone” warrants exercisable into our series A preferred stock for $1.95754 per warrant (or $2,250,000 in the aggregate). The warrants are exercisable for cash for up to 1,149,401 shares of series A preferred stock at an exercise price of $10.11 per share or on a “net-exercise” basis into such lesser number of shares of series A preferred stock by surrendering a portion of the underlying warrant shares, based on the positive difference between the stated warrant exercise price and the initial public offering price per share in this offering, to pay the exercise price. The term of the milestone warrants is three years from the date of issuance.
Effect of Certain Provisions of our Charter and Bylaws and the Delaware Anti-Takeover Statute
Certain provisions of Delaware law, our certificate of incorporation and our bylaws contain provisions that could have the effect of delaying, deferring or discouraging another party from acquiring control of us. These provisions, which are summarized below, may have the effect of discouraging coercive takeover practices and inadequate takeover bids. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms.
No cumulative voting
The Delaware General Corporation Law (“DGCL”) provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless our certificate of incorporation provides otherwise. Our certificate of incorporation and our bylaws do not provide for cumulative voting in the election of directors.
Undesignated preferred stock
The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue one or more series of preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control. These and other provisions may have the effect of deferring hostile takeovers or delaying changes in control or management of our company.
Calling of special meetings of stockholders and action by written consent
Our certificate of incorporation and our bylaws provide that a special meeting of stockholders for any purpose may be called only by our board of directors, chairman of the board of directors, chief executive officer or president and no other persons. Our certificate of incorporation provides that any action required or permitted to be taken by the stockholders of the company must be effected at a duly called annual or special meeting of stockholders and may not be effected by any consent in writing by the stockholders.
Requirements for advance notification of stockholder nominations and proposals
Our bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors. However, our bylaws may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed. These provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
| 93 |
Classified Board of Directors
The provisions in our certificate of incorporation relating to a classified board of directors may have the effect not only of discouraging attempts by others to buy our company, but also of making it more difficult or impossible for existing shareholders to make management changes. A classified board, which is made up of directors elected for staggered terms, while promoting stability in Board membership and management, also moderates the pace of any change in control of our board of directors by extending the time required to elect a majority, effectively requiring action in at least two annual meetings.
Amendment of bylaws
Our board of directors may alter, amend or repeal the bylaws, in whole or in part, or adopt new bylaws. Stockholders may alter, amend, or repeal the bylaws, in whole or in part, or adopt new bylaws by the affirmative vote of the holders of a majority of the shares of capital stock issued and outstanding and entitled to vote at any annual meeting or special meeting, provided that any such alteration, repeal or adoption of new bylaws is stated in the notice to such special meeting.
Election and Removal of Directors
The DGCL provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless our certificate of incorporation provides otherwise. Our certificate of incorporation does not expressly provide for cumulative voting. Directors may be removed, but only for cause, upon the affirmative vote of holders of at least 75% of the voting power of the outstanding shares of our capital stock entitled to vote generally in the election of directors, voting together as a single class. In addition, the certificate of designation pursuant to which a particular series of preferred stock is issued may provide holders of that series of preferred stock with the right to elect additional directors. In addition, under our certificate of incorporation, our board of directors will be divided into three classes of directors, each of which will hold office for a three-year term. The existence of a classified board could delay a successful tender offeror from obtaining majority control of our board of directors, and the prospect of that delay might deter a potential offeror.
Section 203 of the Delaware General Corporation Law
Upon completion of this offering, we will be subject to the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| ● | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; | |
| ● | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or | |
| ● | At or after the time the stockholder became interested, the business combination was approved by our board of directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| ● | any merger or consolidation involving the corporation and the interested stockholder; | |
| ● | any sale, transfer, lease, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; | |
| ● | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| 94 |
| ● | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and | |
| ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Choice of Forum
Our certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of a fiduciary duty owed by our directors, officers or other employees to us or to our stockholders, (iii) any action asserting a claim against us or any director, officer or other employee arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine, in all cases to the fullest extent permitted by law and subject to the court having personal jurisdiction over the indispensable parties named as defendants; provided that these provisions of our certificate of incorporation will not apply to suits brought to enforce a duty or liability created by the Exchange Act, or any other claim for which the federal courts have exclusive jurisdiction. Our certificate of incorporation further provides that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act, unless we consent in writing to the selection of an alternative forum.
Other Limitations on Stockholder Actions
Our bylaws will also impose some procedural requirements on stockholders who wish to:
| ● | make nominations in the election of directors; | |
| ● | propose that a director be removed; or | |
| ● | propose any other business to be brought before an annual or special meeting of stockholders. |
Under these procedural requirements, in order to bring a proposal before a meeting of stockholders, a stockholder must deliver timely notice of a proposal pertaining to a proper subject for presentation at the meeting to our corporate secretary containing, among other things, the following:
| ● | the stockholder’s name and address; | |
| ● | the number of shares beneficially owned by the stockholder and evidence of such ownership; | |
| ● | the names of all persons with whom the stockholder is acting in concert and a description of all arrangements and understandings with those persons; | |
| ● | a description of any agreement, arrangement or understanding reached with respect to shares of our stock, such as borrowed or loaned shares, short positions, hedging or similar transactions; | |
| ● | a description of the business or nomination to be brought before the meeting and the reasons for conducting such business at the meeting; and | |
| ● | any material interest of the stockholder in such business. |
Our bylaws set out the timeliness requirements for delivery of notice.
In order to submit a nomination for our board of directors, a stockholder must also submit any information with respect to the nominee that we would be required to include in a proxy statement, as well as some other information. If a stockholder fails to follow the required procedures, the stockholder’s proposal or nominee will be ineligible and will not be voted on by our stockholders.
Limitations of Liability and Indemnification
See “Certain Relationships and Related Transactions — Indemnification Agreements.”
Exchange Listing
We intend to list our common stock for trading on The Nasdaq Capital Market under the symbol “ANEB.”
Transfer Agent and Registrar
Upon the completion of this offering, the transfer agent and registrar for our common stock will be Continental Stock Transfer & Trust Company. The transfer agent and registrar’s address is 17 Battery Place, 8th Floor, New York, NY 10004.
| 95 |
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has not been a public market for our common stock. Future sales of substantial shares of our common stock, including shares issued upon the exercise of outstanding options, in the public market after our initial public offering, or the possibility of these sales occurring, could cause the prevailing market price for our common stock to fall or impair our ability to raise equity capital in the future.
We will have shares of common stock outstanding immediately after the completion of this offering based on the number of shares outstanding on March 12, 2021 and assuming no exercise of outstanding stock-based awards after such date (or shares if the underwriters exercise their over-allotment option to purchase additional shares in full). Of those shares, the shares of common stock sold in the offering (or shares if the underwriters exercise their over-allotment option to purchase additional shares in full) will be freely transferable without restriction, unless purchased by persons deemed to be our “affiliates” as that term is defined in Rule 144 under the Securities Act. Any shares purchased by an affiliate may not be resold except pursuant to an effective registration statement or an applicable exemption from registration, including an exemption under Rule 144 promulgated under the Securities Act. The remaining shares of common stock to be outstanding immediately following the completion of this offering are “restricted,” which means they were originally sold in offerings that were not registered under the Securities Act. Restricted shares may be sold through registration under the Securities Act or under an available exemption from registration, such as provided through Rule 144, which rules are summarized below. Taking into account the lock-up agreements described below, and assuming the representative of the underwriters does not release any stockholders from the lock-up agreements, the restricted shares of our common stock will be available for sale in the public market as follows:
| ● | shares will be eligible for sale immediately upon completion of this offering; | |
| ● | shares will become eligible for sale, subject to the provisions of Rule 144 or Rule 701, upon the expiration of lock-up agreements not to sell such shares entered into between the underwriter and such stockholders beginning 180 days after the date of this prospectus; and | |
| ● | additional shares will be eligible for sale from time to time thereafter upon expiration of their respective six-month holding periods. |
Rule 144
In general, under Rule 144 of the Securities Act, as in effect on the date of this prospectus, a person (or persons whose shares are aggregated) who has beneficially owned restricted stock for at least six months, will be entitled to sell in any three-month period a number of shares that does not exceed the greater of:
| ● | 1% of the number of shares of common stock then outstanding ( shares immediately after this offering or shares if the underwriters’ over-allotment option to purchase additional shares is exercised in full); or | |
| ● | the average weekly trading volume of our common stock on Nasdaq during the four calendar weeks immediately preceding the date on which the notice of sale is filed with the SEC. |
Subject to the lock-up agreements described above, our affiliates who have beneficially owned shares of our common stock for at least six months, including the holding period of any prior owner other than one of our affiliates, will be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
| ● | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering; and | |
| ● | the average weekly trading volume in our common stock on Nasdaq during the four calendar weeks preceding the date of filing of a Notice of Proposed Sale of Securities Pursuant to Rule 144 with respect to the sale. |
Sales pursuant to Rule 144 are subject to requirements relating to manner of sale, notice and availability of current public information about us. A person (or persons whose shares are aggregated) who is not deemed to be an affiliate of ours for 90 days preceding a sale, and who has beneficially owned restricted stock for at least one year is entitled to sell such shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144. Rule 144 will not be available to any stockholders until we have been subject to the reporting requirements of the Exchange Act for 90 days.
| 96 |
Form S-8 Registration Statement
Following the completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act to register the shares of our common stock that are issuable pursuant to our 2020 Stock Incentive Plan. Shares covered by this registration statement will be eligible for sale in the public markets, subject to vesting restrictions, any applicable lock-up agreements described below and Rule 144 limitations applicable to affiliates.
Rule 701
Rule 701 under the Securities Act, as in effect on the date of this prospectus, permits resale of shares in reliance upon Rule 144 but without compliance with certain restrictions of Rule 144, including the holding period requirement. Most of our employees, executive officers, directors or consultants who purchased shares under a written compensatory plan or contract may be entitled to rely on the resale provisions of Rule 701, but all holders of Rule 701 shares are required to wait until 90 days after the date of this prospectus before selling their shares. However, substantially all Rule 701 shares are subject to lock-up agreements as described below and under “Underwriting” included in this prospectus and will become eligible for sale upon the expiration of the restrictions set forth in those agreements.
Lock-Up Agreements
All of our executive officers, directors and certain of our stockholders have agreed that, without the prior written consent of The Benchmark Company, LLC, as representative of the several underwriters, we and they will not, during the period ending 180 days after the date of this prospectus:
| ● | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exercisable or exchangeable for our common stock; or | |
| ● | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of our common stock; |
whether any transaction described above is to be settled by delivery of shares of our common stock or such other securities, in cash or otherwise. This agreement is subject to certain exemptions, as set forth in the section entitled “Underwriting.”
Registration Rights
In connection with our private placement of series A preferred stock in June 2020, we and 22NW, LP entered into an Investors’ Rights Agreement. Pursuant to the terms of this agreement, 22NW, LP is entitled to “piggyback” registration rights with respect to the registration of the series A preferred stock, including the shares of common stock issuable upon the conversion of the series A preferred stock, under the Securities Act, which we refer to as our registrable securities. If we register any of our securities either for our own account or for the account of other security holders, 22NW, LP is entitled to include its shares in the registration. In the event we register securities in connection with an underwritten offering, the underwriter will have the right to limit the number of shares included in such offering. The registration rights granted under the Investors’ Rights Agreement will terminate with respect to the registrable securities upon the earlier of (i) the date on which the registrable securities may be sold pursuant to Rule 144 of the Securities Act without regard to the volume limitations for sales as provided in Rule 144 or (ii) the third anniversary of this offering. All fees, costs and expenses of registrations under the Investors’ Rights Agreement will be borne by us and all selling expenses, including underwriting discounts and selling commissions, if any, will be borne by 22NW, LP. 22NW, LP has waived its registration rights in connection with the offering to which this prospectus relates.
| 97 |
We are offering the shares of common stock described in this prospectus through the underwriters listed below. Subject to the terms and conditions set forth in the underwriting agreement between us and the underwriters named below, for which The Benchmark Company, LLC is acting as the representative (the “Representative”), we have agreed to sell to the underwriters, and each underwriter has agreed to purchase, severally and not jointly, the number of shares of our common stock listed opposite to its name in the table below.
| Underwriter | Number of Shares | |||
| The Benchmark Company, LLC | ||||
| Total | ||||
Under the terms of the underwriting agreement, the underwriters are committed to purchase, severally and not jointly, all of the shares offered by this prospectus (other than the shares subject to the underwriters’ option to purchase additional shares), if the underwriters buy any of such shares. The underwriters’ obligation to purchase the shares is subject to satisfaction of certain conditions, including, among others, the continued accuracy of representations and warranties made by us in the underwriting agreement, delivery of legal opinions and the absence of any material changes in our assets, business or prospects after the date of this prospectus.
The underwriters initially propose to offer the common stock directly to the public at the public offering price set forth on the front cover page of this prospectus and to certain dealers at such offering price less a concession not to exceed $ per share. After the initial public offering of the shares of our common stock, the offering price and other selling terms may be changed by the underwriters. Sales of shares of our common stock made outside the United States may be made by affiliates of certain of the underwriters.
The shares sold in this offering are expected to be ready for delivery against payment in immediately available funds on or about , 2021, subject to customary closing conditions. The underwriters may reject all or part of any order.
Over-Allotment Option
We have granted to the underwriters an option to purchase up to additional shares of our common stock at the same price to the public, and with the same underwriting discount, as set forth in the table below. The underwriters may exercise this option in whole or in part at any time within 30 days after the date of this prospectus, but only to cover over-allotments, if any. To the extent the underwriters exercise this option, each underwriter will be committed, so long as the conditions of the underwriting agreement are satisfied, to purchase the shares for which they exercise the option.
Discounts and Commissions
The following table shows underwriting discounts and commissions we will pay to the underwriters. These amounts are shown assuming both no exercise and full exercise of the underwriters’ over-allotment option. In addition to the underwriting discount, we have agreed to pay up to $157,500 of the fees and expenses of the underwriters, which may include up to $150,000 of fees and expenses of counsel to the underwriters. The fees and expenses of the underwriters that we have agreed to reimburse are not included in the underwriting discounts and commissions set forth in the table below. We have also agreed with the Representative to grant certain rights of participation and future financing fees associated with offerings undertaken during the nine-month period following this offering. Except as disclosed in this prospectus, the underwriters have not received and will not receive from us any other item of compensation or expense in connection with this offering considered by the Financial Industry Regulatory Authority (“FINRA”) to be underwriting compensation under FINRA Rule 5110. The underwriting discount was determined through an arms’ length negotiation between us and the underwriters.
| Total | ||||||||||||
| Per Share | No Over- Allotment | Over-Allotment | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discounts and commissions to be paid by us: | $ | $ | $ | |||||||||
| Total | $ | $ | $ | |||||||||
| Proceeds, before expenses, to us | $ | $ | $ | |||||||||
| 98 |
We estimate that the total expenses of this offering payable by us, excluding underwriting discounts and commissions, will be approximately $ . This includes $157,500 of fees and expenses of the underwriters.
Stabilization
In accordance with Regulation M under the Exchange Act, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including short sales and purchases to cover positions created by short positions, stabilizing transactions, syndicate covering transactions, penalty bids and passive market making.
| ● | Short positions involve sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares involved in the sales made by the underwriters in excess of the number of shares they are obligated to purchase is not greater than the number of shares that they may purchase by exercising their option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in their option to purchase additional shares. The underwriters may close out any short position by either exercising their option to purchase additional shares or purchasing shares in the open market. | |
| ● | Stabilizing transactions permit bids to purchase the underlying security as long as the stabilizing bids do not exceed a specific maximum price. | |
| ● | Syndicate covering transactions involve purchases of our common stock in the open market after the distribution has been completed to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the underwriters’ option to purchase additional shares. If the underwriters sell more shares than could be covered by the underwriters’ option to purchase additional shares, thereby creating a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. | |
| ● | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. | |
| ● | In passive market making, market makers in our common stock who are underwriters or prospective underwriters may, subject to limitations, make bids for or purchase shares of our common stock until the time, if any, at which a stabilizing bid is made. |
These activities may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result of these activities, the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on Nasdaq or otherwise and, if commenced, may be discontinued at any time.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the Representative will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including civil liabilities under the Securities Act, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
| 99 |
IPO Pricing
Prior to the completion of this offering, there has been no public market for our common stock. The initial public offering price has been negotiated between us and the Representative. Among the factors considered in these negotiations are:
| ● | The information in this prospectus and otherwise available to the underwriters, including our financial information; | |
| ● | the history of, and prospects for, us and the industry in which we compete; | |
| ● | our past and present financial performance; | |
| ● | an assessment of the ability and experience of our management; | |
| ● | the present state of our development and our current financial condition; | |
| ● | the prospects for our future earnings; | |
| ● | the general condition of the economy and the prevailing conditions of the applicable United States securities market at the time of this offering; | |
| ● | previous trading prices for our common stock in the private market and market valuations of publicly traded companies that we and the representative believe to be comparable to us; and | |
| ● | other factors as were deemed relevant. |
We cannot be sure that the initial public offering price will correspond to the price at which the shares of our common stock will trade in the public market following this offering or that an active trading market for the shares of our common stock will develop or continue after this offering.
Lock-Up Agreements
We have agreed that for a period of 180 days after the date of this prospectus, we will not, without the prior written consent of the Representative, which may be withheld or delayed in the Representative’s sole discretion:
| ● | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant for the sale of, lend or otherwise dispose of or transfer, directly or indirectly, any of our common stock or any securities convertible into or exercisable or exchangeable for our common stock, or file any registration statement under the Securities Act with respect to any of the foregoing; or | |
| ● | enter into any swap or other arrangement that transfers to another, in whole or in part, directly or indirectly, any of the economic consequences of ownership of any of our common stock; |
whether any such transaction described above is to be settled by delivery of shares of our common stock or such other securities, in cash or otherwise. The prior sentence will not apply to (i) the shares to be sold pursuant to the underwriting agreement, (ii) any shares of our common stock issued by us upon the exercise of an option or other security outstanding on the date hereof, (iii) such issuances of options or grants of restricted stock or other equity-based awards under our 2020 Stock Incentive Plan and the issuance of shares issuable upon exercise of any such equity-based awards, and (iv) the filing by us of registration statements on Form S-8.
Each of our directors and our executive officers has agreed that for a period ending 180 days after the date of this prospectus, none of them will, without the prior written consent of the Representative which may be withheld or delayed in the Representative’s sole discretion:
| ● | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant for the sale of, lend or otherwise dispose of or transfer, directly or indirectly, any shares of our common stock, or any securities convertible into or exercisable or exchangeable for our common stock owned directly by such director or executive officer or with respect to which such director or executive officer has beneficial ownership; or |
| 100 |
| ● | enter into any swap or other arrangement that transfers to another, in whole or in part, directly or indirectly, any of the economic consequences of ownership of our common stock, whether any such transaction described above is to be settled by delivery of our common stock or such other securities, in cash or otherwise. |
Notwithstanding the prior sentence, subject to applicable securities laws and the restrictions contained in our charter, our directors and executive officers may transfer our securities: (i) pursuant to the exercise or conversion of our securities, including, without limitation, options and warrants, so long as any shares issued upon such exercise are not sold during the lock-up period; (ii) as a bona fide gift or gifts, provided that the donee or donees thereof agree to be bound in writing by the restrictions set forth above; (iii) to any trust for the direct or indirect benefit of such director or executive officer or the immediate family of such director or executive officer, provided that the trustee of the trust agrees to be bound in writing by the restrictions set forth above; (iv) any transfer required under any benefit plans or our charter or bylaws; (v) as required by participants in our 2020 Stock Incentive Plan in order to reimburse or pay federal income tax and withholding obligations in connection with vesting of restricted stock grants or the exercise of stock options or warrants; or (vi) in or in connection with any merger, consolidation, combination or sale of all or substantially all of our assets or in connection with any tender offer or other offer to purchase at least 50% of our common stock.
Other Relationships
The underwriters, including the Representative, and their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. The underwriters may in the future receive customary fees and commissions for these transactions.
In the ordinary course of their various business activities, the underwriters and their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of the issuer. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Electronic Distribution
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members, if any, participating in the offering. The Representative may allocate a number of shares to the underwriters and selling group members, if any, for sale to their online brokerage account holders. Any such allocations for online distributions will be made by the representative on the same basis as other allocations.
Listing
In connection with this offering, we intend to apply to have our common stock listed on The Nasdaq Capital Market under the symbol “ANEB.” There is no assurance, however, that our common stock will be listed on The Nasdaq Capital Market or any other national securities exchange.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company.
| 101 |
Selling Restrictions
No action has been taken in any jurisdiction except the United States that would permit a public offering of our common stock, or the possession, circulation or distribution of this prospectus or any other material relating to us or our common stock in any jurisdiction where action for that purpose is required. Accordingly, the shares may not be offered or sold, directly or indirectly, and neither this prospectus nor any other offering material or advertisements in connection with the shares may be distributed or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45 106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31 103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33 105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State) an offer to the public of any shares of our common stock may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any shares of our common stock may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| ● | to any legal entity which is a qualified investor as defined in the Prospectus Directive; | |
| ● | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representative; or | |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares of our common stock shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive. |
Each person in a Relevant Member State who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with the representative and us that it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representative has been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase or subscribe the shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC (as amended by Directive 2010/73/EU), and includes any relevant implementing measure in the Relevant Member State.
| 102 |
United Kingdom
Each underwriter has, separately and not jointly, represented and agreed that:
| ● | it has not made or will not make an offer of the securities to the public in the United Kingdom within the meaning of section 102B of the Financial Services and Markets Act 2000 (as amended) (“FSMA”), except to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities or otherwise in circumstances which do not require the publication by us of a prospectus pursuant to the Prospectus Rules of the Financial Services Authority; | |
| ● | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000, or FSMA) received by it in connection with the issue or sale of the shares of our common stock in circumstances in which Section 21(1) of the FSMA does not apply to us; and | |
| ● | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of our common stock in, from or otherwise involving the United Kingdom. |
Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. Accordingly, no public distribution, offering or advertising, as defined in CISA, its implementing ordinances and notices, and no distribution to any non-qualified investor, as defined in CISA, its implementing ordinances and notices, shall be undertaken in or from Switzerland, and the investor protection afforded to acquirers of interests in collective investment schemes under CISA does not extend to acquirers of shares.
Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission, or the ASIC, in relation to the offering.
This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001, or the Corporations Act, and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act.
Any offer in Australia of the shares may only be made to persons, the Exempt Investors, who are “sophisticated investors” (within the meaning of section 708(8) of the Corporations Act), “professional investors” (within the meaning of section 708(11) of the Corporations Act) or otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act.
| 103 |
The shares applied for by Exempt Investors in Australia must not be offered for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act. Any person acquiring shares must observe such Australian on-sale restrictions.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Israel
In the State of Israel this prospectus shall not be regarded as an offer to the public to purchase shares of common stock under the Israeli Securities Law, 5728—1968, which requires a prospectus to be published and authorized by the Israel Securities Authority, if it complies with certain provisions of Section 15 of the Israeli Securities Law, 5728—1968, including, inter alia, if: (i) the offer is made, distributed or directed to not more than 35 investors, subject to certain conditions (the “Addressed Investors”); or (ii) the offer is made, distributed or directed to certain qualified investors defined in the First Addendum of the Israeli Securities Law, 5728—1968, subject to certain conditions (the “Qualified Investors”). The Qualified Investors shall not be taken into account in the count of the Addressed Investors and may be offered to purchase securities in addition to the 35 Addressed Investors. We have not and will not take any action that would require us to publish a prospectus in accordance with and subject to the Israeli Securities Law, 5728—1968. We have not and will not distribute this prospectus or make, distribute or direct an offer to subscribe for our securities to any person within the State of Israel, other than to Qualified Investors and up to 35 Addressed Investors.
Qualified Investors may have to submit written evidence that they meet the definitions set out in of the First Addendum to the Israeli Securities Law, 5728—1968. In particular, we may request, as a condition to be offered securities, that Qualified Investors will each represent, warrant and certify to us and/or to anyone acting on our behalf: (i) that it is an investor falling within one of the categories listed in the First Addendum to the Israeli Securities Law, 5728—1968; (ii) which of the categories listed in the First Addendum to the Israeli Securities Law, 5728—1968 regarding Qualified Investors is applicable to it; (iii) that it will abide by all provisions set forth in the Israeli Securities Law, 5728—1968 and the regulations promulgated thereunder in connection with the offer to be issued securities; (iv) that the securities that it will be issued are, subject to exemptions available under the Israeli Securities Law, 5728—1968: (a) for its own account; (b) for investment purposes only; and (c) not issued with a view to resale within the State of Israel, other than in accordance with the provisions of the Israeli Securities Law, 5728—1968; and (v) that it is willing to provide further evidence of its Qualified Investor status. Addressed Investors may have to submit written evidence in respect of their identity and may have to sign and submit a declaration containing, inter alia, the Addressed Investor’s name, address and passport number or Israeli identification number.
Hong Kong
The contents of this document have not been reviewed or approved by any regulatory authority in Hong Kong. This document does not constitute an offer or invitation to the public in Hong Kong to acquire shares. Accordingly, unless permitted by the securities laws of Hong Kong, no person may issue or have in its possession for the purposes of issue, this document or any advertisement, invitation or document relating to the shares, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong other than in relation to shares which are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” (as such term is defined in the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) (“SFO”) and the subsidiary legislation made thereunder); or in circumstances which do not result in this document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32, Laws of Hong Kong) (“CO”); or which do not constitute an offer or an invitation to the public for the purposes of the SFO or the CO. The offer of the shares is personal to the person to whom this document has been delivered, and a subscription for shares will only be accepted from such person. No person to whom a copy of this document is issued may issue, circulate or distribute this document in Hong Kong, or make or give a copy of this document to any other person. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this document, you should obtain independent professional advice.
| 104 |
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor pursuant to Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (“SFA”), (ii) to a relevant person (as defined in Section 275(2) of the SFA), or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased pursuant to an offer made in reliance on Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or | |
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor; |
shares, debentures and units of shares, and debentures of that corporation, or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for six months after that corporation or that trust has acquired the shares under Section 275 except:
| (1) | to an institutional investor or to a relevant person (as defined in Section 275(2) of the SFA), or any person pursuant to Section 275(1A) of the SFA (in the case of that corporation) or Section 276(4)(i)(B) of the SFA (in the case of that trust); | |
| (2) | where no consideration is or will be given for the transfer; or | |
| (3) | where the transfer is by operation of law. |
| 105 |
Olshan Frome Wolosky LLP, New York, New York, will pass upon the validity of the shares of our common stock being offered by this prospectus as our counsel in connection with this offering. The underwriters have been represented in connection with this offering by Faegre Drinker Biddle & Reath LLP.
The balance sheet of Anebulo Pharmaceuticals, Inc. as of June 30, 2020, and the related statements of operations, changes in convertible preferred stock, common stock and stockholders’ deficit, and cash flows for the period from April 23, 2020 (inception) to June 30, 2020, have been audited by EisnerAmper LLP, independent registered public accounting firm, as stated in their report which is incorporated herein, which report includes an explanatory paragraph about the existence of substantial doubt concerning the Company’s ability to continue as a going concern. Such financial statements have been incorporated herein in reliance on the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1, which includes exhibits, schedules and amendments, under the Securities Act with respect to the shares of our common stock we are offering pursuant to this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is contained in exhibits to the registration statement, as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the contract, agreement or other document summarized, but are not complete descriptions of all terms of those contracts, agreements or other documents. If we filed any of those contracts, agreements or other documents as an exhibit to the registration statement, you may read the contract, agreement or other document itself for a complete description of its terms. Each statement in this prospectus relating to a contract, agreement or other document filed as an exhibit is qualified in all respects by the filed exhibit. When we complete this offering, we will become subject to the information and reporting requirements of the Exchange Act and, in accordance with law, we will be required to file annual, quarterly and special reports, proxy statements and other information with the SEC.
You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at www.sec.gov. We also maintain a website at www.anebulo.com, at which, following the completion of this offering, you may access our annual, quarterly and special reports, proxy statements and other information free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on, or that can be accessed through, our website does not constitute part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only. Investors should not rely on any such information in deciding whether to purchase our common stock.
| 106 |
| Page | |
| Anebulo Pharmaceuticals, Inc | |
| As of June 30, 2020 and for the period from April 23, 2020 (inception) to June 30, 2020 | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Balance Sheet as of June 30, 2020 | F-3 |
| Statement of Operations for the period from April 23, 2020 (inception) to June 30, 2020 | F-4 |
| Statement of Convertible Preferred Stock, Common Stock and Stockholders’ Deficit for the period from April 23, 2020 (inception) to June 30, 2020 | F-5 |
| Statement of Cash Flows for the period from April 23, 2020 (inception) to June 30, 2020 | F-6 |
| Notes to Financial Statements | F-7 |
| Page | |
| Anebulo Pharmaceuticals, Inc | |
| As of December 31, 2020 (unaudited) and June 30, 2020 (audited) and for the six months ended December 31, 2020 (unaudited) | |
| Balance Sheets as of December 31, 2020 (unaudited) and June 30, 2020 (audited) | F-16 |
| Statement of Operations for the six months ended December 31, 2020 (unaudited) | F-17 |
| Statement of Convertible Preferred Stock, Common Stock and Stockholders’ Deficit for the six months ended December 31, 2020 (unaudited) | F-18 |
| Statement of Cash Flows for the six months ended December 31, 2020 (unaudited) | F-19 |
| Notes to Financial Statements | F-20 |
| F-1 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Anebulo Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheet of Anebulo Pharmaceuticals, Inc. (the “Company”) as of June 30, 2020, and the related statements of operations, convertible preferred stock, common stock and stockholders’ deficit, and cash flows for period from April 23, 2020 (inception) to June 30, 2020, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2020, and the results of its operations and its cash flows for the period from April 23, 2020 (inception) to June 30, 2020, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company incurred, and it anticipates it will continue to incur, losses and generate negative operating cash flows and as such will require significant additional funds to continue its development activities. These factors raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ EisnerAmper LLP
We have served as the Company’s auditor since 2020.
EISNERAMPER LLP
Iselin, New Jersey
December 18, 2020
| F-2 |
Balance Sheet
| June 30, 2020 | ||||
| Assets | ||||
| Current assets: | ||||
| Cash and cash equivalents | $ | 3,024,980 | ||
| Receivable - related party | 3,500 | |||
| Total current assets | 3,028,480 | |||
| Total assets | $ | 3,028,480 | ||
| Liabilities, convertible preferred stock and stockholders’ deficit | ||||
| Current liabilities: | ||||
| Accrued expenses | $ | 22,579 | ||
| Promissory notes - related party | 201,286 | |||
| Total current liabilities | 223,865 | |||
| Total liabilities | 223,865 | |||
| Commitments and contingencies | ||||
| Series A convertible preferred stock, $0.0001 par value; 1,490,651 shares authorized; 341,250 shares issued and outstanding at June 30, 2020 | 2,975,752 | |||
| Stockholders’ deficit: | ||||
| Common stock, $0.001 par value; 3,800,000 shares authorized; 2,000,000 shares issued and outstanding at June 30, 2020 | 2,000 | |||
| Additional paid-in capital | 1,500 | |||
| Accumulated deficit | (174,637 | ) | ||
| Total stockholders’ deficit | (171,137 | ) | ||
| Total liabilities, convertible preferred stock, and stockholders’ deficit | $ | 3,028,480 | ||
The accompanying notes are an integral part of these financial statements.
| F-3 |
Statement of Operations
| For the period from April 23, 2020 (inception) | ||||
| to June 30, 2020 | ||||
| Operating expenses: | ||||
| Research and development | $ | 150,000 | ||
| General and administrative | 23,351 | |||
| Total operating expenses | 173,351 | |||
| Other expense: | ||||
| Interest expense | (1,286 | ) | ||
| Loss from operations before taxes | (174,637 | ) | ||
| Tax expense | - | |||
| Net loss | $ | (174,637 | ) | |
| Weighted average common shares outstanding, basic and diluted | 2,000,000 | |||
| Net loss per share, basic and diluted | $ | (0.09 | ) | |
The accompanying notes are an integral part of these financial statements.
| F-4 |
Statement of Convertible Preferred Stock, Common Stock and Stockholders’ Deficit
Series A Convertible Preferred Stock | Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balance at April 23, 2020 | - | $ | - | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Issuance of common stock | - | - | 2,000,000 | 2,000 | 1,500 | - | 3,500 | |||||||||||||||||||||
| Issuance of Series A convertible preferred stock, net of issuance costs of $24,248 | 341,250 | 2,975,752 | - | - | - | - | - | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (174,637 | ) | (174,637 | ) | |||||||||||||||||||
| Balance at June 30, 2020 | 341,250 | $ | 2,975,752 | 2,000,000 | $ | 2,000 | $ | 1,500 | $ | (174,637 | ) | $ | (171,137 | ) | ||||||||||||||
The accompanying notes are an integral part of these financial statements.
| F-5 |
Statement of Cash Flows
| For the period from April 23, 2020 (inception) | ||||
| to June 30, 2020 | ||||
| Cash flows from operating activities: | ||||
| Net loss | $ | (174,637 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Promissory notes accrued interest | 1,286 | |||
| Changes in operating assets and liabilities: | ||||
| Accrued expenses | 22,579 | |||
| Net cash used in operating activities | (150,772 | ) | ||
| Cash flows from financing activities: | ||||
| Proceeds from issuance of promissory notes to related party | 200,000 | |||
| Proceeds from issuance of Series A convertible preferred stock | 3,000,000 | |||
| Payment of issuance costs on Series A convertible preferred stock | (24,248 | ) | ||
| Net cash provided by financing activities | 3,175,752 | |||
| Net increase in cash, cash equivalents and restricted cash | 3,024,980 | |||
| Cash and cash equivalents, beginning of year | - | |||
| Cash and cash equivalents, end of year | $ | 3,024,980 | ||
| Supplemental Disclosure of Noncash Investing and Financing Activities: | ||||
| Proceeds due from issuance of common stock to founder | $ | 3,500 | ||
The accompanying notes are an integral part of these financial statements.
| F-6 |
NOTES TO FINANCIAL STATEMENTS
Note 1. Organization, Principal Activities, and Basis of Presentation
Anebulo Pharmaceuticals, Inc. (“Company”) was founded on April 23, 2020, as a Delaware corporation. The Company is a clinical stage biotechnology company focused on developing and commercializing new treatments for patients suffering from cannabinoid overdose and addiction. The Company’s principal operations are located in Lakeway, Texas.
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position, results of operations and cash flows for the period presented.
From inception, the Company has devoted substantially all of its efforts to raising capital and acquiring licensing rights to its drug product. The Company has determined that it has one operating and reporting segment. The Company has one lead product candidate, ANEB-001, under development, which was licensed from Vernalis (R&D) Ltd in May 2020 (“License Agreement”), as fully described in Note 7.
Note 2. Liquidity and Going Concern
The financial statements have been prepared as though the Company will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The Company has incurred operating losses and negative cash flows from operations since inception. As of June 30, 2020, the Company has an accumulated deficit of $174,637. Management expects to continue to incur operating losses and negative cash flows from operations within one year after the date the financial statements are issued. In addition, as more fully described in Note 7, the Company is subject to milestone payments associated with a License Agreement. Management believes the Company has access to capital through private placements, collaboration agreements, and other potential equity funding transactions, as well as potential debt capital raises.
Through June 2020, the Company raised $3,200,000 of funding through the sale of its Series A Convertible Preferred Stock (“Series A Preferred”) and issuance of two promissory notes. The Company will need to raise additional capital in order to continue to fund operations, including milestone obligations under its License Agreement. The Company believes that it will be able to obtain additional capital through equity financings or other arrangements to fund operations; however, there can be no assurance that such additional financing, if available, can be obtained on terms acceptable to the Company. If the Company is unable to obtain such additional financing, future operations would need to be scaled back or discontinued.
Accordingly, these factors raise substantial doubt about the Company’s ability to continue as a going concern for a period of at least one year after the date the financial statements are available to be issued. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
| F-7 |
Note 3. Summary of Significant Accounting Policies
Use of Estimates and Assumptions
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, and expenses, and the disclosure of contingent assets and liabilities as of and during the reporting period. The Company bases its estimates and assumptions on historical experience when available and on various factors that it believes to be reasonable under the circumstances. The Company assesses estimates on an ongoing basis; however, actual results could materially differ from those estimates. The most significant estimates are related to legal expenses.
Risk and Uncertainties
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash and cash equivalents. Periodically, the Company may maintain deposits in financial institutions in excess of government insured limits. Management believes that the Company is not exposed to significant credit risk as the Company’s deposits are held at financial institutions that management believes to be of high credit quality, and the Company has not experienced any losses on these deposits.
The Company operates in an industry that is subject to intense competition, government regulations and rapid technological change. Operations are subject to significant risk and uncertainties including financial, operational, technological, regulatory, and other risks, including potential risk of business failure.
In March 2020, the World Health Organization declared the global novel coronavirus disease 2019 (Covid-19) outbreak a pandemic. As of June 30, 2020, the Company’s operations have not been significantly impacted by the Covid-19 outbreak. However, the Company cannot at this time predict the specific extent, duration, or full impact that the Covid-19 outbreak will have on its financial condition and operations, including ongoing and planned clinical trials.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents.
Fair Value of Financial Instruments
The Company follows the guidance prescribed by FASB Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements (“ASC 820”), which establishes the following hierarchy that prioritizes the inputs used to measure fair value:
| ● | Level 1 Inputs: Unadjusted quoted prices in active markets for identical assets or liabilities accessible to the reporting entity at the measurement date. | |
| ● | Level 2 Inputs: Other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the asset or liability. | |
| ● | Level 3 Inputs: Unobservable inputs for the asset or liability used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at measurement date. |
| F-8 |
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement). Fair value is defined as the proceeds that would be received for an asset or the exit price that would be paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants on the measurement date.
Convertible Preferred Stock
The Company has classified Series A Preferred as temporary equity in the accompanying balance sheets due to certain change in control events that are outside of the Company’s control, including sale or transfer of control of the Company, as holders of the Series A Preferred could cause redemption of the shares in these situations.
Research and Development Costs
Research and development costs are charged to expense as incurred. Payments for these activities will be based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the financial statements as prepaid or accrued research and development. Research and development activities may consist of salaries and benefits, contract services, materials and supplies, stock-based compensation expense, depreciation of equipment, and other outside expenses. As of June 30, 2020, the only research and development expense the Company incurred was the initial payment for the licensing agreement. (See note 7).
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the Company’s financial statements and tax returns. Deferred tax assets and liabilities are determined based upon the differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities and for loss and credit carryforwards, using enacted tax rates expected to be in effect in the year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance if it is more likely than not that these assets may not be realized. The Company determines whether it is more likely than not that a tax position will be sustained upon examination. If it is not more likely than not that a position will be sustained, none of the benefit attributable to the position is recognized. The tax benefit to be recognized for any tax position that meets the more-likely-than-not recognition threshold is calculated as the largest amount that is more than 50% likely of being realized upon resolution of the contingency. The Company accounts for interest and penalties related to uncertain tax positions as part of its provision for income taxes. The Company is a calendar year (December 31st) tax filer.
Stock-based Compensation
The Company recognizes compensation expense for awards to employees and nonemployees based on the grant date fair value of stock-based awards on a straight-line basis over the period during which an award holder provides service in exchange for the award. For awards subject to performance conditions, the Company recognizes compensation expense using an accelerated recognition method over the remaining service period when management determines that achievement of the milestone is probable. Management evaluates when the achievement of a performance-based milestone is probable based on the expected satisfaction of the performance conditions as of the reporting date. The fair value is calculated using the Black-Scholes option pricing model. The Company recognizes stock-based award forfeitures as they occur rather than estimating a forfeiture rate in accordance with the guidance per Accounting Standard Update (“ASU”) No. 2016-09.
| F-9 |
In June 2018, the FASB issued ASU No. 2018-07, “Compensation—Stock Compensation”, which expands the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees, except for specific exceptions. This ASU is effective for annual or any interim periods beginning after December 15, 2019. The Company adopted this standard on April 23, 2020 (inception).
As of June 30, 2020, the Company had not issued any stock awards. Subsequently, in October 2020, the Company issued 163,750 stock awards to its Chief Executive Officer (“CEO”) (see Note 13 for further details).
Leases
In February 2016, the FASB issued ASU No. 2016-02, “Leases” (“ASC 842”) to enhance the transparency and comparability of financial reporting related to leasing arrangements. Under this new lease standard, most leases are required to be recognized on the balance sheet as right-of-use assets and lease liabilities. Disclosure requirements have been enhanced with the objective of enabling financial statement users to assess the amount, timing, and uncertainty of cash flows arising from leases. Prior to January 1, 2019, U.S. GAAP did not require lessees to recognize assets and liabilities related to operating leases on the balance sheet. The new standard establishes a right-of-use (“ROU”) model that requires a lessee to recognize a ROU asset and corresponding lease liability on the balance sheet for all leases with a term longer than 12 months. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement as well as the reduction of the right-of-use asset.
This ASU is effective for nonpublic reporting companies for interim and annual periods beginning after December 15, 2021, with early adoption permitted, and must be adopted using a modified retrospective approach. The Company has adopted the standard effective April 23, 2020 (inception). The Company has elected to apply (i) the practical expedient which allows the Company to not separate lease and non-lease components, for new leases entered into after adoption and (ii) the short-term lease exemption for all leases with an original term of less than 12 months, for purposes of applying the recognition and measurements requirements in the new standard.
At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on specific facts and circumstances, the existence of an identified asset(s), if any, and the Company’s control over the use of the identified asset(s), if applicable. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of future lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company will utilize the incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term on an amount equal to the lease payments in a similar economic environment.
Operating leases are recognized on the balance sheet as ROU lease assets, lease liabilities current and lease liabilities non-current. Fixed rents are included in the calculation of the lease balances while variable costs paid for certain operating and pass-through costs are excluded. Lease expense is recognized over the expected term on a straight-line basis.
As of June 30, 2020, the Company had not entered into any leases. Subsequently, in August 2020, the Company entered into a one-year sub-lease for office space in Lakeway, Texas, from a related party.
Loss Per Share
Series A Preferred participates on a one-for-one basis with common stock in the distribution of dividends, if and when declared by the Board of Directors.
| F-10 |
Since the Company has reported a loss for the period from April 23, 2020 (inception) to June 30, 2020, therefore, no income was allocated to Series A Preferred. Basic and diluted net loss per share are the same because the impact of Series A Preferred would be anti-dilutive and have been excluded from the computation of diluted weighted-average shares outstanding.
Subsequent Events
The Company has evaluated and, as necessary, made changes to these financial statements for subsequent events through December 18, 2020, the date these financial statements were available to be issued. All subsequent events that provided additional evidence about conditions existing at the date of the statements of financial position were incorporated into the financial statements (see Note 13 for further detail).
Reverse Stock Split
On June 18, 2020, the Company implemented a 1-for-1.75 reverse stock split of the Company’s common stock. All share and per share data shown in the accompanying financial statements and related notes have been retroactively revised to reflect the reverse stock split. Shares of common stock underlying outstanding stock options and other equity instruments were proportionately reduced and the respective exercise prices, if applicable, were proportionately increased in accordance with the terms of the agreements governing such securities. Shares of common stock reserved for issuance upon the conversion of the Company’s Preferred Stock were proportionately reduced and the respective conversion prices were proportionately increased.
Recently Issued Accounting Pronouncements
The Company considers the applicability and impact of all ASUs. ASUs not discussed below were assessed and determined to be either not applicable or are expected to have minimal impact on the financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which simplifies the accounting for income taxes by removing certain exceptions to the general principles in the existing guidance for income taxes and making other minor improvements. The amendments are effective for annual reporting periods beginning after December 15, 2020 with early adoption permitted. The Company is currently evaluating the impact of adopting this new accounting guidance.
Note 4. Fair Value of Measurements
The Company’s financial instruments consist of cash and cash equivalents, accounts payable, and accrued expenses. The carrying amount of cash and cash equivalents, accounts payable, accrued expenses and promissory note to a related party is considered a reasonable estimate of fair value due to the short-term nature of those instruments.
The Company’s financial assets which are measured at fair value on a recurring basis were comprised of cash and cash equivalents of $3,024,980 at June 30, 2020, based on Level 1 inputs.
Note 5. Accrued Expenses
Accrued expenses of $22,579 at June 30, 2020 consisted of accrued legal fees.
Note 6. Promissory Notes
On May 28, 2020 and June 18, 2020, the Company issued an aggregate of $200,000 in promissory notes (“2020 Notes”) to a related party investor. The annual interest rate on the 2020 Notes is a fixed rate of 8.0%.
| F-11 |
All accrued and unpaid interest and principal on the promissory note issued on May 28, 2020, is due and payable on demand of the Company on or after the date on which the Company consummates an equity financing (or series of equity financings having materially similar terms and conditions) pursuant to which the Company sells and issues shares of preferred stock for total aggregate gross proceeds of at least $2,500,000 (the “Maturity Date”). As of the date of these financial statements, the related party investor has not yet demanded repayment of the note.
All accrued and unpaid interest and principal on the promissory note issued on June 18, 2020 shall be due and payable on demand of the Company on June 17, 2023.
For the period from April 23, 2020 (inception) through June 30, 2020, the Company had interest expense of $1,286.
Note 7. License Agreement
In May 2020, the Company licensed certain intellectual property, know-how and clinical trial data from Vernalis (R&D) Ltd. The initial consideration in exchange for the license was $150,000 and is recorded as research and development expense in the statement of operations. The license term shall continue unless and until terminated for cause or insolvency, sixty day written notice, or until such time as all royalties and other sums cease to be payable in accordance with the terms of the agreement.
The Company is required to pay development milestone payments related to clinical trials and granting of marketing authorization ranging from $350,000 to $3,000,000, up to a total development milestone payment of $29,900,000, and sales milestone payments of $10,000,000 and $25,000,000, in the first year when cumulative annual net sales of licensed product exceeds $500,000,000 and $1,000,000,000, respectively. The Company is also required to pay single-digit royalties on product sales over the term of the contract. The Company has determined that none of the milestone payments are considered probable as of June 30, 2020 and therefore no liability has been recorded.
Note 8. Income Taxes
The Company’s effective tax rate differs from the statutory federal tax rate as presented in the following table:
| For the period from April 23, 2020 (inception) | ||||
| to June 30, 2020 | ||||
| U.S. federal statutory tax rate | 21.0 | % | ||
| Valuation Allowance | (21.0 | )% | ||
| Total | 0.0 | % | ||
As of June 30, 2020, the Company was domiciled in Texas, and due to the losses generated and no revenues, it incurred no federal or state tax.
| F-12 |
The tax effect of the temporary differences that give rise to the significant portions of the deferred tax assets and liabilities is presented below:
| For the period from April 23, 2020 (inception) | ||||
| to June 30, 2020 | ||||
| Deferred tax assets: | ||||
| Net operating losses | $ | 34,927 | ||
| Total gross deferred tax asset | 34,927 | |||
| Valuation allowance | (34,927 | ) | ||
| Net deferred tax asset | $ | - | ||
There is no current tax expense and deferred tax expense for the period from April 23, 2020 (inception) to June 30, 2020.
Net Operating Losses (“NOLs”) arising in tax years ending after December 31, 2017 and before January 1, 2021 are carried forward indefinitely. The Company has no income tax expense due to operating losses incurred for period from April 23, 2020 (inception) to June 30, 2020. The Company has provided a valuation allowance for the full amount of the deferred tax assets as, based on all available evidence, it is considered more likely than not that all the recorded deferred tax assets will not be realized in a future period.
At June 30, 2020, the Company has federal NOLs of $174,637. Certain of these federal net operating loss carryforwards may be subject to Internal Revenue Code Section 382 or similar provisions, which impose limitations on their utilization amounts.
Realization of the future tax benefits is dependent on many factors, including the Company’s ability to generate taxable income. Under the provisions of the Internal Revenue Code, certain substantial changes in the Company’s ownership, including a sale of the Company or significant changes in ownership due to sales of equity, may have limited, or may limit in the future, the amount of net operating loss carryforwards that could be used annually to offset future taxable income. The Company has not completed a study to assess whether a change of control has occurred or whether there have been multiple changes of control since the Company’s formation. As a result, the Company is not able to estimate the effect of the change in control, if any, on the Company’s ability to utilize net operating loss and research and development credit carryforwards in the future.
Since the Company was formed in April 2020 and has elected to be on a calendar year tax filer, the Company has not filed any tax returns in the United States, nor in the State of Texas. The Company is not currently under examination by the IRS or any other jurisdictions for any tax years.
Entities are also required to evaluate, measure, recognize and disclose any uncertain income tax provisions taken on their income tax returns. The Company has analyzed its tax positions and has concluded that as of June 30, 2020, the Company had no uncertain tax positions. The Company has elected to recognize interest and penalties related to income tax matters as a component of income tax expense, of which no interest or penalties were recorded for the period from April 23, 2020 (inception) to June 30, 2020.
| F-13 |
Note 9. Series A Convertible Preferred Stock
In June 2020, the Company authorized the sale and issuance of up to 1,490,651 shares of Series A Preferred. The Series A Preferred financing was structured so that 341,250 shares would be issued at the first closing to one investor (“Initial Investor”) at $8.7912 per share (“First Closing”) and up to 1,149,401 shares at $10.11 per share could be issued upon the exercise of certain warrants (“Milestone Warrants”).
Upon achieving certain development milestones by the Company and being certified by the Board of Directors, the Company has the obligation to issue and the Initial Investor plus one designated additional investor (“Additional Investor”) have the right and obligation to purchase Milestone Warrants to purchase 638,556 and 510,845 shares of Series A Preferred, respectively. The Milestone Warrants will have a purchase price of $1.95754 per share of the additional 1,149,401 shares of Series A Preferred for total proceeds of $2,250,000 and the right to purchase the additional 1,149,401 shares of Series A Preferred at $10.11 per share. The term of the Milestone Warrants will be three years from the date of issuance.
On June 18, 2020, the Company issued 341,250 shares of Series A Preferred for gross cash proceeds of $3,000,000. Issuance costs paid totaled $24,248.
As of June 30, 2020, the requisite development milestones were not yet achieved, and therefore no Milestone Warrants nor additional shares of Series A Preferred have been issued.
The Company determined the obligation of the Company and the rights and obligations of the initial Series A Preferred shareholder and the one designated additional investor to purchase Milestone Warrants does not meet the definition of a freestanding financial instrument as it is not separable from the Series A Preferred issued in June 2020.
As of June 30, 2020, the rights and preferences of the Series A Preferred are as follows:
Conversion - Each share of Series A Preferred may be converted at any time, at the option of the holder, into shares of common stock, subject to the applicable conversion rate as determined by dividing the original issue price by the conversion price. The initial conversion price for the Series A Preferred issued at the First Closing is $8.7912, however, it may be adjusted for certain dilutive events. The initial conversion price for the Series A Preferred issued upon the exercise of the Milestone Warrants will be $10.11, however, it may be adjusted for certain dilutive events. The Series A Preferred automatically converts into shares of common stock at a 1:1 conversion ratio at the earlier of the closing of a public offering of the Company’s securities at any price per share or at the election of the holders of at least a majority of the then-outstanding shares of Series A Preferred.
If the Initial Investor or any of its affiliates that may have received a portion of the shares from the Initial Closing, fails to purchase the designated Milestone Warrant upon the achievement of the development milestones, then all of shares from the Initial Closing still held by the Initial Investor and any of its affiliates will automatically convert into shares of Common Stock at a 1:1 conversion.
Dividends - Series A Preferred shareholders shall first receive, or simultaneously receive, a dividend if declared on any other class or series of capital stock.
Voting Rights - Preferred Stock and common stockholders vote together as one class on an as converted basis. Common stock voting rights on certain matters are subject to the powers, preferences, and rights of the Preferred Stock. Holders are entitled to vote on all matters and shall have the number of votes equal to the number of shares of common stock into which the shares of Preferred Stock held by such holder are then convertible. Certain actions such as mergers, acquisition, liquidation, dissolution, wind up of business, and deemed liquidation events, must be approved by the holders of at least a majority of the then-outstanding shares of Series A Preferred.
Liquidation Preference - Upon liquidation, dissolution, or winding up of business, the Preferred Stock holders are entitled to receive a liquidation preference in priority to holders of common stock equal to the original Series A Preferred issue price plus any accrued but unpaid dividends if that amount is greater than what it would have received had their shares been converted to common stock. If assets available for distribution are insufficient to satisfy the liquidation payment to holders in full, assets available for distribution will be allocated among holders based on their pro rata shareholdings. When holders are satisfied in full, any excess assets available for distribution will be allocated ratably among common stockholders based on their pro rata shareholdings. The liquidation preference as of June 30, 2020 is $3,000,000.
| F-14 |
Redemption – Other than as described in Note 3, the Series A Preferred is not redeemable.
Note 10. Common Stock
The Company authorized the sale and issuance of up to 3,800,000 shares of common stock. One related party investor owns 100% of the 2,000,000 outstanding shares of common stock as of June 30, 2020. As of June 30, 2020, the related party investor owed the Company $3,500, for the purchase of these shares. Subsequently, in September 2020, the related party investor paid the Company for these shares.
As of June 30, 2020, the Company had reserved 275,000 shares of common stock for the 2020 Stock Incentive Plan, 341,250 shares of common stock for the conversion of Series A Preferred, and 1,149,401 shares of common stock for the exercise of Milestone Warrants.
As of June 30, 2020, the rights of the common stockholders are as follows:
Voting Rights - The holders of the common stock are entitled to one vote for each share of common stock. The voting, dividend, and liquidation rights of the holders of the Common Stock are subject to and qualified by the rights, powers, and preferences of the holders of the Series A Preferred.
Dividends - The Corporation shall not declare, pay or set aside any dividends on shares of any other class or series of capital stock of the Corporation (other than dividends on shares of Common Stock payable in shares of Common Stock) unless the holders of the Series A Preferred Stock then outstanding shall first receive, or simultaneously receive, a dividend on each outstanding share of Series A Preferred stock then outstanding.
Liquidation Preference - In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Company, after the payment or provision for payment of all debts and liabilities of the Company and all preferential amounts to which the holders of Preferred Stock are entitled with respect to the distribution of assets in liquidation, the holders of common stock shall be entitled to share ratably in the remaining assets of the Company available for distribution.
Note 11. Stock Incentive Plan
In June 2020, the Board of Directors adopted the 2020 Stock Incentive Plan, which provided for the grant of qualified incentive stock options and nonqualified stock options or other awards to the Company’s employees, officers, directors, advisors, and outside consultants for the purchase of up to 275,000 shares of the Company’s common stock. Other awards include restricted stock, restricted stock units, stock appreciation rights and other stock-based awards. Other stock-based awards are awards valued in whole or in part by reference to, or are otherwise based on, shares of common stock. Stock options generally vest over a four-year period and expire ten years from the date of grant. No stock-based awards were issued for the period from April 23, 2020 (inception) to June 30, 2020.
Note 12. Subsequent Events
The Company has completed an evaluation of all subsequent events through December 18, 2020, the date these financial statements were available to be issued. The Company has concluded that no subsequent events have occurred that require disclosure except as disclosed below and in Note 3 and 10.
| ● | In July 2020, the Company hired a CEO, under a two-year employment agreement, which will be automatically extended for successive one-year periods, unless either party gives notice of non-extension to the other party no later than 30 days prior to the expiration date. CEO’s compensation will substantially be derived from the Other Stock-Based Awards of 163,750 shares of common stock issued under the Company’s 2020 Stock Incentive Plan and will vest based on reaching certain performance targets. | |
| ● | The Company entered into a one-year sublease for office space in Lakeway, Texas with a related party. The lease commenced in August 2020 with the initial term set to expire in August 2021. This office lease does not have a renewal option. Annual rent for this lease is $14,434. | |
| ● | In October 2020, the Company entered into an agreement with a third-party contract manufacturing organization to begin manufacturing and conducting stability studies on ANEB-001 drug substance, ANEB-001 drug product and placebo. The cost of these contracts are approximately $973,000. |
| F-15 |
Balance Sheets
| December 31, 2020 | June 30, 2020 | |||||||
| (unaudited) | (audited) | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 2,480,003 | $ | 3,024,980 | ||||
| Receivable - related party | - | 3,500 | ||||||
| Prepaid expenses and other current assets | 28,855 | - | ||||||
| Total current assets | 2,508,858 | 3,028,480 | ||||||
| Deferred offering costs | 101,651 | - | ||||||
| Total assets | $ | 2,610,509 | $ | 3,028,480 | ||||
| Liabilities, convertible preferred stock and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 84,477 | $ | - | ||||
| Accrued expenses | 59,946 | 22,579 | ||||||
| Promissory notes - related party | 209,352 | 201,286 | ||||||
| Total current liabilities | 353,775 | 223,865 | ||||||
| Commitments and contingencies | ||||||||
| Series A convertible preferred stock, $0.0001 par value; 1,490,651 shares authorized; 341,250 shares issued and outstanding at December 31, 2020 and June 30, 2020 | 2,975,752 | 2,975,752 | ||||||
| Stockholders’ deficit: | ||||||||
| Common stock, $0.001 par value; 3,800,000 shares authorized; 2,163,750, and 2,000,000 shares issued and outstanding at December 31, 2020 and June 30, 2020, respectively | 2,164 | 2,000 | ||||||
| Additional paid-in capital | 38,438 | 1,500 | ||||||
| Accumulated deficit | (759,620 | ) | (174,637 | ) | ||||
| Total stockholders’ deficit | (719,018 | ) | (171,137 | ) | ||||
| Total liabilities, convertible preferred stock, and stockholders’ deficit | $ | 2,610,509 | $ | 3,028,480 | ||||
The accompanying notes are an integral part of these financial statements.
| F-16 |
Statement of Operations
| Six months ended | ||||
| December 31, 2020 | ||||
| (unaudited) | ||||
| Operating expenses: | ||||
| Research and development | $ | 190,268 | ||
| General and administrative | 386,649 | |||
| Total operating expenses | 576,917 | |||
| Other expense: | ||||
| Interest expense | (8,066 | ) | ||
| Loss from operations before taxes | (584,983 | ) | ||
| Income tax expense | - | |||
| Net loss | $ | (584,983 | ) | |
| Weighted average common shares outstanding, basic and diluted | 2,136,162 | |||
| Net loss per share, basic and diluted | $ | (0.27 | ) | |
The accompanying notes are an integral part of these financial statements.
| F-17 |
Statement of Convertible Preferred Stock, Common Stock and Stockholders’ Deficit
(unaudited)
Series A Convertible Preferred | Additional | Total | ||||||||||||||||||||||||||
| Stock | Common Stock | Paid-in | Accumulated | Stockholders’ | ||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balance at June 30, 2020 (audited) | 341,250 | $ | 2,975,752 | 2,000,000 | $ | 2,000 | $ | 1,500 | $ | (174,637 | ) | $ | (171,137 | ) | ||||||||||||||
| Issuance of restricted common stock | - | - | 163,750 | 164 | (164 | ) | - | - | ||||||||||||||||||||
| Stock-based compensation expense | - | - | - | - | 37,102 | - | 37,102 | |||||||||||||||||||||
| Net loss | - | - | - | - | - | (584,983 | ) | (584,983 | ) | |||||||||||||||||||
| Balance at December 31, 2020 | 341,250 | $ | 2,975,752 | 2,163,750 | $ | 2,164 | $ | 38,438 | $ | (759,620 | ) | $ | (719,018 | ) | ||||||||||||||
The accompanying notes are an integral part of these financial statements.
| F-18 |
Statement of Cash Flows
| Six months ended | ||||
| December 31, 2020 | ||||
| Cash flows from operating activities: | (unaudited) | |||
| Net loss | $ | (584,983 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Stock-based compensation | 37,102 | |||
| Promissory notes accrued interest | 8,066 | |||
| Changes in operating assets and liabilities: | ||||
| Receivable - related party | 3,500 | |||
| Prepaid expenses and other current assets | (28,855 | ) | ||
| Deferred offering costs | (101,651 | ) | ||
| Accounts payable | 84,477 | |||
| Accrued expenses | 37,367 | |||
| Net cash used in operating activities | (544,977 | ) | ||
| Net decrease in cash, cash equivalents and restricted cash | (544,977 | ) | ||
| Cash and cash equivalents, beginning of period | 3,024,980 | |||
| Cash and cash equivalents, end of the period | $ | 2,480,003 | ||
The accompanying notes are an integral part of these financial statements.
| F-19 |
NOTES TO FINANCIAL STATEMENTS
Note 1. Organization, Principal Activities, and Basis of Presentation
Anebulo Pharmaceuticals, Inc. (“the Company”) was founded on April 23, 2020, as a Delaware corporation. The Company is a clinical stage biotechnology company focused on developing and commercializing new treatments for patients suffering from cannabinoid overdose and addiction. The Company’s principal operations are located in Lakeway, Texas.
The accompanying interim unaudited financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position, results of operations and cash flows for the period presented. The accompanying interim unaudited financial statements should be read in conjunction with the audited financial statements and notes thereto as of June 30, 2020 and for the period from April 23, 2020 (inception) to June 30, 2020.
From inception, the Company has devoted substantially all of its efforts to raising capital and acquiring licensing rights to its drug product. The Company has determined that it has one operating and reporting segment. The Company has one lead product candidate, ANEB-001, under development, which was licensed from Vernalis (R&D) Ltd in May 2020 (“License Agreement”), as described in Note 7.
Note 2. Liquidity and Going Concern
Through December 31, 2020, the Company has raised $3,200,000 of funding through the sales of its Series A Convertible Preferred Stock (“Series A Preferred”) and the issuance of two promissory notes. As of December 31, 2020, the Company had accumulated deficit of $759,620 and cash and cash equivalents of $2,480,003. The Company’s ability to continue as a going concern is highly contingent on the ability to raise additional capital for ongoing research and development and clinical trials as the Company expects to continue incurring losses for the foreseeable future.
Management believes the Company has access to capital through private placements, corporate collaborations, and other potential equity funding transactions, as well as potential debt capital raises. The Company is currently evaluating these alternatives to fund its future operations.
Management cannot provide assurance that sufficient required additional funding will become available on commercially acceptable terms to continue the Company’s ongoing and planned research and development and clinical trials. If the Company is unable to secure required additional funding, this could affect future business activities and continuing development that is critical to the Company’s future operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying interim financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Note 3. Summary of Significant Accounting Policies
Basis of Presentation
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions. The accompanying interim unaudited financial statements for the period presented have been prepared on substantially the same basis as the Company’s annual financial statements for the fiscal year ended June 30, 2020. In the opinion of the Company’s management, these financial statements reflect all adjustments, consisting of only normal, recurring adjustments, necessary to fairly state the Company’s financial position, results of operations and cash flows. The preparation of these interim unaudited financial statements requires the Company to make estimates and judgments that affect the amounts reported in the financial statements and the accompanying notes. The Company’s actual results may differ from these estimates under different assumptions or conditions.
| F-20 |
Use of Estimates and Assumptions
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, and expenses, and the disclosure of contingent assets and liabilities as of and during the reporting period. The Company bases its estimates and assumptions on historical experience when available and on various factors that it believes to be reasonable under the circumstances. The Company assesses estimates on an ongoing basis; however, actual results could materially differ from those estimates. The most significant estimates are related to research and development contracts, legal expenses and stock-based compensation.
Risk and Uncertainties
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash and cash equivalents. Periodically, the Company may maintain deposits in financial institutions in excess of government insured limits. Management believes that the Company is not exposed to significant credit risk as the Company’s deposits are held at financial institutions that management believes to be of high credit quality, and the Company has not experienced any losses on these deposits.
The Company operates in an industry that is subject to intense competition, government regulations and rapid technological change. Operations are subject to significant risk and uncertainties including financial, operational, technological, regulatory, and other risks, including potential risk of business failure.
In March 2020, the World Health Organization declared the global novel coronavirus disease 2019 (COVID-19) outbreak a pandemic. As of December 31, 2020, the Company’s operations have not been significantly impacted by the COVID-19 outbreak. However, the Company cannot at this time predict the specific extent, duration, or full impact that the COVID-19 outbreak will have on its financial condition and operations, including ongoing and planned clinical trials.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents.
Fair Value of Financial Instruments
The Company follows the guidance prescribed by FASB Accounting Standards Codification (“ASC”) Topic 820, Fair Value Measurements (“ASC 820”), which establishes the following hierarchy that prioritizes the inputs used to measure fair value:
| ● | Level 1 Inputs: Unadjusted quoted prices in active markets for identical assets or liabilities accessible to the reporting entity at the measurement date. |
| ● | Level 2 Inputs: Other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the asset or liability. |
| ● | Level 3 Inputs: Unobservable inputs for the asset or liability used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at measurement date. |
The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement). Fair value is defined as the proceeds that would be received for an asset or the exit price that would be paid to transfer a liability in the principal or most advantageous market in an orderly transaction between market participants on the measurement date.
Convertible Preferred Stock
The Company has classified its Series A Preferred securities as temporary equity in the accompanying balance sheets due to certain change in control events that are outside of the Company’s control, including sale or transfer of control of the Company, as holders of the Series A Preferred could cause redemption of the shares in these situations.
Deferred Offering Costs
In conjunction with a possible initial public offering (“IPO”) of the Company’s common stock, costs incurred related to the IPO are capitalized as deferred equity issuance costs in other non-current assets until the IPO is completed or the potential IPO is abandoned. If the Company completes an IPO, these costs will be offset against proceeds received; or if the IPO does not occur, they will be expensed. Offering costs include direct and incremental costs related to the offering such as legal fees and related costs associated with the proposed IPO. As of December 31, 2020, the Company recorded deferred IPO offering costs of $101,651.
| F-21 |
Research and Development Costs
Research and development costs are charged to expense as incurred. Payments for these activities will be based on the terms of the individual arrangements, which may differ from the pattern of costs incurred, and are reflected in the interim unaudited financial statements as prepaid or accrued research and development. Research and development activities may consist of salaries and benefits, contract services, materials and supplies, stock-based compensation expense, depreciation of equipment, and other outside expenses.
Stock-Based Compensation
The Company recognizes stock-based compensation expense related to stock options granted to employees and non-employees based on the estimated fair value of the awards on the date of grant. The Company estimates the grant date fair value, and the resulting stock-based compensation expense, for stock options that only have service vesting requirements or performance-based vesting requirements without market conditions using the Black-Scholes option-pricing model. The grant date fair value of the stock-based awards with service vesting requirements is generally recognized on a straight-line basis over the requisite service period, which is generally the vesting period of the respective awards. Determining the appropriate amount to expense for performance-based awards based on the achievement of stated goals requires judgment. The estimate of expense is revised periodically based on the probability of achieving the required performance targets and adjustments are made as appropriate. The cumulative impact of any revisions is reflected in the period of change. If any applicable financial performance goals are not met, no compensation cost is recognized, and any previously recognized compensation cost is reversed.
The Black-Scholes option-pricing model requires the use of highly subjective assumptions, which determine the fair value of stock-based awards. These assumptions include:
Expected term - Our expected term represents the period that the stock-based awards are expected to be outstanding and is determined using the simplified method (based on the mid-point between the vesting date and the end of the contractual term). For stock-based awards granted to non-employees, the expected term represents the contractual term of the award.
Common stock price - The Board of directors estimates the fair value of common stock. Given the absence of a public trading market for its common stock, and in accordance with the American Institute of Certified Public Accountants’ Practice Guide, Valuation of Privately Held-Company Equity Securities Issued as Compensation, the board of directors exercises reasonable judgment and considers a number of objective and subjective factors to determine its best estimate of the fair value of the common stock, as further described below under “Common stock valuations.”
Expected volatility - The Company is a privately held company and did not have any trading history for its common stock and the expected volatility was estimated using weighted-average measures of implied volatility and the historical volatility of its peer group of companies for a period equal to the expected life of the stock options. The peer group of publicly traded biopharmaceutical companies was chosen based on their similar size, stage in the life cycle or area of specialty.
Risk-free interest rate - The risk-free interest rate is based on the rates paid on securities issued by the U.S. Treasury with a term approximating the expected life of the stock options.
Expected dividend - The Company has never paid, and does not anticipate paying, cash dividends on its common stock. Therefore, the expected dividend yield was assumed to be zero.
In addition to the Black-Scholes assumptions, The Company adopted ASU 2016-09 in June 2020 and as a result, the Company has made an entity-wide accounting policy election to account for pre-vesting award forfeitures when they occur.
| F-22 |
Leases
In February 2016, the FASB issued ASU No. 2016-02, “Leases” (“ASC 842”) to enhance the transparency and comparability of financial reporting related to leasing arrangements. Under this new lease standard, most leases are required to be recognized on the balance sheet as right-of-use assets and lease liabilities. Disclosure requirements have been enhanced with the objective of enabling financial statement users to assess the amount, timing, and uncertainty of cash flows arising from leases. Prior to January 1, 2019, U.S. GAAP did not require lessees to recognize assets and liabilities related to operating leases on the balance sheet. The new standard establishes a right-of-use (“ROU”) model that requires a lessee to recognize a ROU asset and corresponding lease liability on the balance sheet for all leases with a term longer than 12 months. Leases will be classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the income statement as well as the reduction of the right-of-use asset.
This ASU is effective for non-public reporting companies for interim and annual periods beginning after December 15, 2021, with early adoption permitted, and must be adopted using a modified retrospective approach. The Company has adopted the standard effective April 23, 2020 (inception). The Company has elected to apply (i) the practical expedient which allows the Company to not separate lease and non-lease components, for new leases entered into after adoption and (ii) the short-term lease exemption for all leases with an original term of less than 12 months, for purposes of applying the recognition and measurements requirements in the new standard.
At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on specific facts and circumstances, the existence of an identified asset(s), if any, and the Company’s control over the use of the identified asset(s), if applicable. Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of future lease payments over the expected lease term. The interest rate implicit in lease contracts is typically not readily determinable. As such, the Company will utilize the incremental borrowing rate, which is the rate incurred to borrow on a collateralized basis over a similar term on an amount equal to the lease payments in a similar economic environment.
Operating leases are recognized on the balance sheet as ROU lease assets, lease liabilities current and lease liabilities non-current. Fixed rents are included in the calculation of the lease balances while variable costs paid for certain operating and pass-through costs are excluded. Lease expense is recognized over the expected term on a straight-line basis.
In August 2020, the Company entered into a one-year sub-lease for office space in Lakeway, Texas, from a related party and recorded rent expense of $5,413 for the six months ended December 31, 2020. Remaining payments due under the lease total $8,420.
Loss Per Share
The Company’s Series A Preferred securities participate on a one-for-one basis with common stock in the distribution of dividends, if and when declared by the Board of Directors.
Since the Company has reported a loss for the six months ended December 31, 2020, therefore, no income was allocated to the Company’s Series A Preferred securities. Basic and diluted net loss per share are the same because the impact of Series A Preferred would be anti-dilutive and has been excluded from the computation of diluted weighted-average shares outstanding.
Reverse Stock Split
On June 18, 2020, the Company implemented a 1-for-1.75 reverse stock split of the Company’s common stock. All share and per share data shown in the accompanying interim unaudited financial statements and related notes have been retroactively revised to reflect the reverse stock split. Shares of common stock underlying outstanding stock options and other equity instruments were proportionately reduced and the respective exercise prices, if applicable, were proportionately increased in accordance with the terms of the agreements governing such securities. Shares of common stock reserved for issuance upon the conversion of the Company’s preferred stock were proportionately reduced and the respective conversion prices were proportionately increased.
Recently Issued Accounting Pronouncements
The Company considers the applicability and impact of all ASUs. ASUs not discussed below were assessed and determined to be either not applicable or are expected to have minimal impact on the financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which simplifies the accounting for income taxes by removing certain exceptions to the general principles in the existing guidance for income taxes and making other minor improvements. The amendments are effective for annual reporting periods beginning after December 15, 2020 with early adoption permitted. The Company is currently evaluating the impact of adopting this new accounting guidance.
| F-23 |
Note 4. Fair Value of Measurements
The Company’s financial instruments consist of cash and cash equivalents, accounts payable, and accrued expenses. The carrying amount of cash and cash equivalents, accounts payable, accrued expenses and the promissory note to a related party are considered a reasonable estimate of fair value due to the short-term nature of those instruments.
The Company’s financial assets which are measured at fair value on a recurring basis were comprised of cash and cash equivalents of $2,480,003 at December 31, 2020 and $3,024,980 at June 30, 2020, based on Level 1 inputs. As of December 31, 2020, the Company did not have any Level 2 or Level 3 assets or liabilities.
Note 5. Accrued Expenses
Accrued expenses of $59,946 at December 31, 2020 consisted of accrued research and development consulting of $59,141 and accrued payroll of $805. Accrued expenses of $22,579 at June 30, 2020 consisted of accrued legal expenses.
Note 6. Promissory Notes
On May 28, 2020 and June 18, 2020, the Company issued promissory notes (“2020 Notes”) for $175,000 and $25,000, respectively, to a related party investor. The annual interest rate on the 2020 Notes is a fixed rate of 8.0%.
Total principal and accrued and unpaid interest of $183,170 for the promissory note issued on May 28, 2020, is due and payable on demand by the related party investor of the Company. As of the date of these interim unaudited financial statements, the related party investor has not yet demanded repayment of the note.
Total principal and accrued and unpaid interest of $26,182 for the promissory note issued on June 18, 2020 shall be due and payable on demand by the related party investor of the Company on June 17, 2023.
For the six months ended December 31, 2020, the Company recorded interest expense of $8,066.
The carrying value of the 2020 Notes at December 31, 2020 and June 30, 2020 was $209,352 and $201,286, respectively.
Note 7. License Agreement
In May 2020, the Company licensed certain intellectual property, know-how and clinical trial data from Vernalis (R&D) Ltd. The Company is required to make cash payments upon reaching certain development milestones (“Development Milestones”) related to clinical trials, granting of marketing authorization and sales milestones. The Company is also required to pay single-digit royalties on product sales over the term of the contract. During the six months ended December 31, 2020, the Company did not reach any of the Development Milestones and therefore did not record any additional license expense under this agreement.
Note 8. Series A Convertible Preferred Stock
In June 2020, the Company authorized the sale and issuance of up to 1,490,651 shares of Series A Preferred. The Series A Preferred financing was structured so that 341,250 shares would be issued at the first closing to one investor (“Initial Investor”) at $8.7912 per share (“First Closing”) and up to 1,149,401 shares at $10.11 per share could be issued upon the exercise of certain warrants (“Milestone Warrants”).
Upon achieving certain development milestones by the Company and being certified by the Board of Directors, the Company has the obligation to issue and the Initial Investor plus one designated additional investor (“Additional Investor”) have the right and obligation to purchase Milestone Warrants to purchase 127,711 and 1,021,690 shares of Series A Preferred, respectively and as amended. The Milestone Warrants will have a purchase price of $1.95754 per share of the additional 1,149,401 shares of Series A Preferred for total proceeds of $2,250,000 and the right to purchase the additional 1,149,401 shares of Series A Preferred at $10.11 per share. The term of the Milestone Warrants will be three years from the date of issuance.
On June 18, 2020, the Company issued 341,250 shares of Series A Preferred for gross cash proceeds of $3,000,000. Issuance costs paid in cash totaled $24,248.
| F-24 |
As of December 31, 2020, the requisite development milestones were not yet achieved, and therefore no Milestone Warrants or additional shares of Series A Preferred have been issued.
The Company determined the obligation of the Company, the rights and obligations of the initial Series A Preferred shareholder and the one designated additional investor to purchase Milestone Warrants does not meet the definition of a freestanding financial instrument as it is not separable from the Series A Preferred issued in June 2020.
As of December 31, 2020, the rights and preferences of the Series A Preferred are as follows:
Conversion - Each share of Series A Preferred may be converted at any time, at the option of the holder, into shares of common stock, subject to the applicable conversion rate as determined by dividing the original issue price by the conversion price. The initial conversion price for the Series A Preferred issued at the First Closing is $8.7912, however, it may be adjusted for certain dilutive events. The initial conversion price for the Series A Preferred issued upon the exercise of the Milestone Warrants will be $10.11, however, it may be adjusted for certain dilutive events. The Series A Preferred automatically converts into shares of common stock at a 1:1 conversion ratio at the earlier of the closing of a public offering of the Company’s securities at any price per share or at the election of the holders of at least a majority of the then-outstanding shares of Series A Preferred.
If the Initial Investor or any of its affiliates that may have received a portion of the shares from the Initial Closing, fails to purchase the designated Milestone Warrant upon the achievement of the development milestones, then all of shares from the Initial Closing still held by the Initial Investor and any of its affiliates will automatically convert into shares of Common Stock at a 1:1 conversion.
Dividends - Series A Preferred shareholders shall first receive, or simultaneously receive, a dividend if declared on any other class or series of capital stock.
Voting Rights - Preferred Stock and common stockholders vote together as one class on an as converted basis. Common stock voting rights on certain matters are subject to the powers, preferences, and rights of the Preferred Stock. Holders are entitled to vote on all matters and shall have the number of votes equal to the number of shares of common stock into which the shares of Preferred Stock held by such holder are then convertible. Certain actions such as mergers, acquisition, liquidation, dissolution, wind up of business, and deemed liquidation events, must be approved by the holders of at least a majority of the then-outstanding shares of Series A Preferred.
Liquidation Preference - Upon liquidation, dissolution, or winding up of business, the Preferred Stockholders are entitled to receive a liquidation preference in priority to holders of common stock equal to the original Series A Preferred issue price plus any accrued but unpaid dividends if that amount is greater than what it would have received had their shares been converted to common stock. If assets available for distribution are insufficient to satisfy the liquidation payment to holders in full, assets available for distribution will be allocated among holders based on their pro rata shareholdings. When holders are satisfied in full, any excess assets available for distribution will be allocated ratably among common stockholders based on their pro rata shareholdings. The liquidation preference as of December 31, 2020 is $3,000,000.
Redemption – Other than as described in Note 3, the Series A Preferred is not redeemable.
Note 9. Common Stock
The Company authorized the sale and issuance of up to 3,800,000 shares of common stock. One related party investor owns 2,000,000 shares of common stock outstanding as of December 31, 2020. As of June 30, 2020, the related party investor owed the Company $3,500, for the purchase of these shares, which was paid in September 2020.
In September 2020, the Company awarded 163,750 shares of restricted common stock to its Chief Executive Officer (“CEO”) under the 2020 Stock Incentive Plan (“2020 Stock Plan”) at a grant date fair value of $0.65 per share. The restrictions are subject to the satisfaction of certain performance targets and vesting requirements pursuant to the award and employment agreement. The restricted common stock has voting and dividend rights, and therefore all 163,750 shares of restricted common stock are considered issued and outstanding as of December 31, 2020.
As of December 31, 2020, the Company had reserved 111,250 shares of common stock for the 2020 Stock Plan, 341,250 shares of common stock for the conversion of Series A Preferred, and 1,149,401 shares of common stock for the conversions of Series A Preferred from the exercise of future Milestone Warrants.
| F-25 |
As of December 31, 2020, the rights of the common stockholders are as follows:
Voting Rights - The holders of the common stock are entitled to one vote for each share of common stock. The voting, dividend, and liquidation rights of the holders of the Common Stock are subject to and qualified by the rights, powers, and preferences of the holders of the Series A Preferred.
Dividends - The Corporation shall not declare, pay or set aside any dividends on shares of any other class or series of capital stock of the Corporation (other than dividends on shares of Common Stock payable in shares of Common Stock) unless the holders of the Series A Preferred Stock then outstanding shall first receive, or simultaneously receive, a dividend on each outstanding share of Series A Preferred stock then outstanding.
Liquidation Preference - In the event of any voluntary or involuntary liquidation, dissolution or winding up of the Company, after the payment or provision for payment of all debts and liabilities of the Company and all preferential amounts to which the holders of Preferred Stock are entitled with respect to the distribution of assets in liquidation, the holders of common stock shall be entitled to share ratably in the remaining assets of the Company available for distribution.
Note 10. Stock Incentive Plan
In June 2020, the Board of Directors adopted the 2020 Stock Plan, which provided for the grant of qualified incentive stock options and nonqualified stock options or other awards to the Company’s employees, officers, directors, advisors, and outside consultants for the purchase of up to 275,000 shares of the Company’s common stock. Other awards include restricted stock, restricted stock units, stock appreciation rights and other stock-based awards. Other stock-based awards are awards valued in whole or in part by reference to, or are otherwise based on, shares of common stock. Stock options generally vest over a four-year period and expire ten years from the date of grant. As of December 31, 2020, there are 111,250 shares available to be granted under the 2020 Stock Plan.
Note 11. Stock-Based Compensation
As of December 31, 2020, the Company has not issued any stock option awards.
In September 2020, the Company awarded 163,750 shares of restricted common stock to its CEO, at a grant date fair value of $0.65 per share. The restrictions are subject to the satisfaction of certain performance targets and vesting requirements pursuant to the award and employment agreement.
In the event of a change in control of our company, the CEO will be entitled to the vesting of 50% of any stock-based awards granted but not yet vested prior to the change in control event not less than six months after the change in control event, provided the CEO remains employed by our company. If the change in control event is an initial public offering, the CEO will be entitled to the full vesting of any stock-based awards.
For the six months ended December 31, 2020, 13,644 shares vested and the Company recorded stock-based compensation expense of $37,102 in general and administrative expenses.
As of December 31, 2020, unrecognized stock-based compensation expense associated with the restricted common stock totaled $69,336.
Note 12. Subsequent Events
The Company has completed an evaluation of all subsequent events through March 12, 2021, the date these interim unaudited financial statements were available to be issued. The Company has concluded that no subsequent events have occurred that require disclosure, with the exception of the following:
| ● | On January 1, 2021, the Company hired a chief financial officer, under an at-will employment agreement. Any termination by the Company, or the by the Executive, shall be communicated by a 30-day written notice to the other party. The chief financial officer’s compensation consists of a base salary only. | |
| ● | On February 11, 2021, the Company entered into an agreement with a third-party clinical research organization to conduct a Phase 2 proof-of-concept trial for cannabinoid overdose in the fourth calendar quarter of 2021 with the anticipation of completing the trial by the first calendar quarter of 2022. The total cost of the agreement is approximately €1,450,758 or $1,760,000. |
***
| F-26 |
Shares

Anebulo Pharmaceuticals, Inc.
Common Stock
PROSPECTUS
The Benchmark Company
, 2021
Through and including , 2021 (the 25th day after the date of this prospectus), all dealers that buy, sell or trade shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotments or subscription.
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all estimated expenses and costs to be paid solely by us, other than estimated underwriting discounts and commissions, in connection with the issuance and distribution of the securities registered hereby. All amounts shown are estimates except for the SEC registration fee, the FINRA filing fee and The Nasdaq Capital Market (“Nasdaq”) listing fee:
| Amount to be Paid | ||||
| SEC registration fee | * | |||
| FINRA filing fee | * | |||
| Nasdaq listing fee | * | |||
| Blue sky fees and expenses | * | |||
| Printing and engraving expenses | * | |||
| Legal fees and expenses | * | |||
| Accounting fees and expenses | * | |||
| Transfer agent and registrar fees | * | |||
| Miscellaneous fees and expenses | * | |||
| Total | $ | * | ||
| * | To be provided by amendment. |
Item 14. Indemnification of Directors and Officers
We are a Delaware corporation and are governed by the Delaware General Corporation Law, or the DGCL. Section 145(a) of the DGCL provides that a Delaware corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no cause to believe his or her conduct was unlawful.
Section 145(b) of the DGCL provides that a Delaware corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that such person acted in any of the capacities set forth above, against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if he or she acted under similar standards, except that no indemnification may be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the court in which such action or suit was brought shall determine that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to be indemnified for such expenses which the Court of Chancery or such other court shall deem proper.
Section 145 of the DGCL further provides that: (i) to the extent that a former or present director or officer of a corporation has been successful in the defense of any action, suit or proceeding referred to in subsections (a) and (b) or in the defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by him or her in connection therewith; (ii) indemnification provided for by Section 145 shall not be deemed exclusive of any other rights to which the indemnified party may be entitled; and (iii) the corporation may purchase and maintain insurance on behalf of any present or former director, officer, employee or agent of the corporation or any person who at the request of the corporation was serving in such capacity for another entity against any liability asserted against such person and incurred by him or her in any such capacity or arising out of his or her status as such, whether or not the corporation would have the power to indemnify him or her against such liabilities under Section 145.
| II-1 |
In addition, the proposed form of Underwriting Agreement (to be filed by amendment) is expected to provide for indemnification of our directors and officers by the underwriters against certain liabilities.
Article Eighth of our certificate of incorporation authorizes us to provide for the indemnification of officers, directors and third parties acting on our behalf to the fullest extent permissible under Delaware law.
We intend to enter into indemnification agreements with our directors, executive officers and others prior to the completion of this offering, in addition to indemnification provided for in our bylaws, and intend to enter into indemnification agreements with any new directors and executive officers in the future.
We have purchased and intend to maintain insurance on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
See also the undertakings set forth in response to Item 17 herein.
Item 15. Recent Sales of Unregistered Securities
On June 18, 2020, we received gross proceeds of $3.0 million from a private placement of our series A preferred stock (the “Private Placement”), convertible into 341,250 shares of our common stock, pursuant to the terms of a Securities Purchase Agreement with 22NW, LP, an institutional accredited investor affiliated with Aron R. English, a director of our company. The series A preferred stock is convertible into shares of common stock automatically upon the closing of this offering. The conversion price of the series A preferred stock is $8.7912 per share. The conversion price is subject to adjustment if, at any time prior to conversion of the shares, we issue in a financing additional shares of common stock or other equity or equity-linked securities at a purchase, conversion or exercise price less than $8.7912 per share. In any such case, we have agreed to issue additional shares of series A preferred stock to the investors so that the effective purchase price per share in the Private Placement is reduced by a weighted-average anti-dilution percentage that takes into account both the lower per share purchase, conversion or exercise price and the number of such additional shares issued at the lower price. No adjustment will be made, however, in respect of shares of common stock or stock options issued to employees, directors or consultants, or in connection with acquisitions of other corporations or strategic collaborations approved by our board of directors.
As part of the Private Placement, 22NW, LP and Mr. English, individually, further agreed under the Securities Purchase Agreement to purchase upon the achievement of certain corporate events “milestone” warrants for $1.95754 per warrant (or $2,250,000 in the aggregate). The warrants are exercisable for cash for up to 1,149,401 shares of series A preferred stock at an exercise price of $10.11 per share or on a “net exercise” basis into such lesser number of shares of series A preferred stock by surrendering a portion of the underlying warrant shares, based on the positive difference between the stated warrant exercise price and the initial public offering price per share in this offering, to pay the exercise price. The warrants must be purchased upon our achievement of (i) a filing with the FDA of an investigational new drug application or the making of an analogous regulatory filing in any foreign jurisdiction, whichever is earlier, and (ii) an arrangement by us to produce the active pharmaceutical ingredient of ANEB-001 in amounts sufficient to facilitate the consummation of a trial pursuant to such regulatory filing. The milestone warrants may also be purchased at any time at the option of 22NW, LP and Mr. English.
The sales and issuances of securities in the transactions described above were not registered under the Securities Act of 1933, as amended, in reliance upon the exemption from registration provided by Section 4(a)(2) thereof and Regulation D promulgated thereunder, which exempts transactions by an issuer not involving any public offering.
| II-2 |
Item 16. Exhibits and Financial Statement Schedules
Exhibit Number |
Description | |
| 1.1* | Form of Underwriting Agreement | |
| 3.1 | Certificate of Incorporation of Anebulo Pharmaceuticals, Inc. | |
| 3.2 | By-laws of Anebulo Pharmaceuticals, Inc. | |
| 3.3* | Form of Amended and Restated Certificate of Incorporation of Anebulo Pharmaceuticals, Inc. (to be effective upon the closing of this offering) | |
| 3.4* | Form of Amended and Restated By-laws of Anebulo Pharmaceuticals, Inc. (to be effective upon the closing of this offering) | |
| 4.1* | Specimen Stock Certificate for Common Stock | |
| 5.1* | Opinion of Olshan Frome Wolosky LLP | |
| 10.1 | Series A Preferred Stock Purchase Agreement, dated June 18, 2020, between Anebulo Pharmaceuticals, Inc. and 22NW, LP | |
| 10.2 | Right of First Refusal and Co-Sale Agreement, dated June 18, 2020, between Anebulo Pharmaceuticals, Inc. and 22NW, LP | |
| 10.3 | Investors’ Rights Agreement, dated June 18, 2020, between Anebulo Pharmaceuticals, Inc. and 22NW, LP | |
| 10.4# | License Agreement, dated May 26, 2020, between Vernalis (R&D) Limited and Anebulo Pharmaceuticals, Inc. | |
| 10.5† | Employment Agreement, dated July 21, 2020, between Daniel Schneeberger and Anebulo Pharmaceuticals, Inc. | |
| 10.6† | Anebulo Pharmaceuticals, Inc. 2020 Stock Incentive Plan, and Form of Award Agreement thereunder | |
| 10.7* | Simple Promissory Note, dated May 28, 2020, by Anebulo Pharmaceuticals, Inc. to Joseph F. Lawler in the principal amount of $175,000 | |
| 10.8* | Simple Promissory Note, dated June 18, 2020, by Anebulo Pharmaceuticals, Inc. to Joseph F. Lawler in the principal amount of $25,000 | |
| 10.9* | Form of Indemnification Agreement between Anebulo Pharmaceuticals, Inc. and each of its directors | |
| 10.10* | Consultancy Agreement, dated July 15, 2020, between Anebulo Pharmaceuticals, Inc. and Traxeus Pharma Services Limited | |
| 14.1* | Code of Ethics and Business Conduct | |
| 14.2* | Code of Ethics for the CEO and Senior Financial Officers | |
| 21.1* | List of subsidiaries | |
| 23.1* | Consent of Olshan Frome Wolosky LLP (included in Exhibit 5.1) | |
| 23.2* | Consent of EisnerAmper LLP | |
| 24.1 | Power of Attorney (included on signature page of the registration statement) |
| † | Compensatory plan or agreement. |
| * | To be filed by amendment. |
| # | Certain of the schedules and attachments to this exhibit have been omitted pursuant to Regulation S-K, Item 601(a)(5). The registrant hereby undertakes to provide further information regarding such omitted materials to the Securities and Exchange Commission upon request. |
| (b) | Financial Statement Schedules |
All financial statement schedules are omitted because the information called for is not required or is shown either in the financial statements or in the notes thereto.
Item 17. Undertakings.
(a) The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
(b) Insofar as indemnification by the Registrant for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the provisions described in Item 14 or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(c) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(l) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-3 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Lakeway, State of Texas, on the day of , 2021.
| ANEBULO PHARMACEUTICALS, INC. | ||
| By: | ||
| Name: | Daniel Schneeberger, M.D. | |
| Title: | Chief Executive Officer | |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Daniel Schneeberger, M.D. and Rex Merchant, and each one of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement and to sign any registration statement for the same offering covered by this registration statement that is to be effective upon filing pursuant to Rule 462 promulgated under the Securities Act of 1933, as amended, and all post-effective amendments thereto, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each one of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| Signature | Title | Date | ||
| Joseph F. Lawler, M.D., Ph.D. | Founder and Director | , 2021 | ||
| Chief Executive Officer (Principal | ||||
| Daniel Schneeberger, M.D. | Executive Officer) | , 2021 | ||
| Chief Financial Officer (Principal | ||||
| Rex Merchant | Financial and Accounting Officer) | , 2021 | ||
| Jason M. Aryeh | Director | , 2021 | ||
| Aron R. English | Director | , 2021 | ||
| Kenneth Lin, M.D. | Director | , 2021 | ||
| Karah Parschauer | Director | , 2021 |
| II-4 |